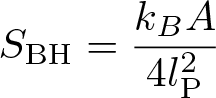Penicillin
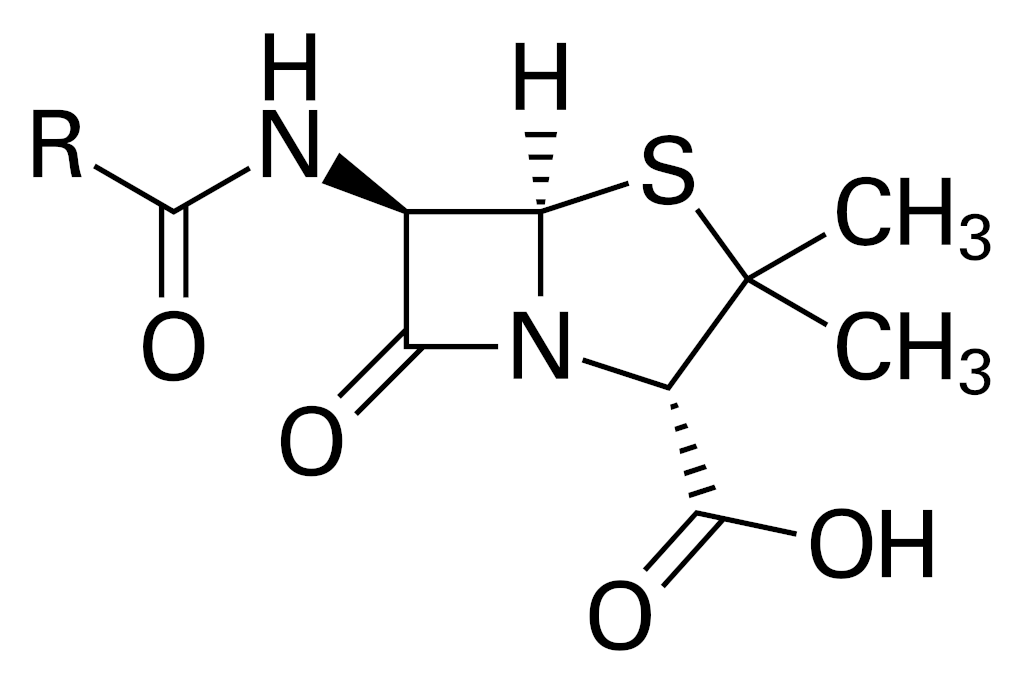
Discovered by: Alexander Fleming at St. Mary's Hospital in London was the first to show that Penicillium rubens had antibacterial properties between 1928-1929.
What is it? A group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens.
Why is it important? Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
Bayes' Theorem
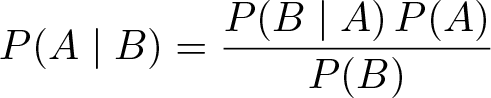
Discovered by: Thomas Bayes in 1763.
What is it? In probability theory and statistics, Bayes’ theorem describes the probability of an event, based on prior knowledge of conditions that might be related to the event.
Why is it important? The seed that grew into the modern field of Bayesian statistics. Sir Harold Jeffreys wrote that Bayes’ theorem “is to the theory of probability what the Pythagorean theorem is to geometry.”
Coenzyme A
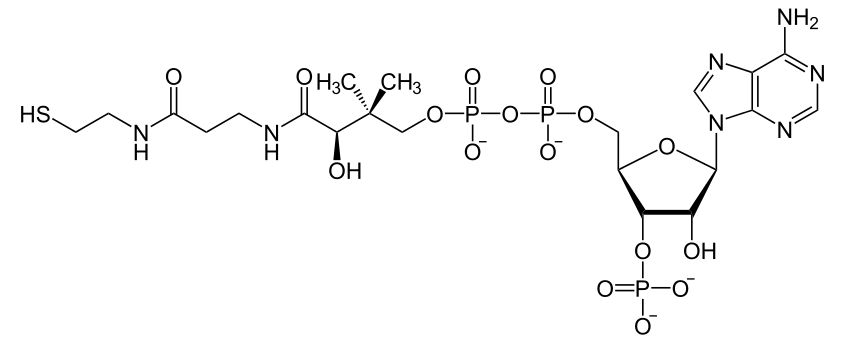
Discovered by: Fritz Lipmann, awarded the 1953 Nobel Prize in Physiology or Medicine "for his discovery of co-enzyme A and its importance for intermediary metabolism".
What is it? A coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle.
Why is it important? Used in the body for fatty acid synthesis, energy production and glucose regulation.
Chlorine

Discovered by: First studied in detail in 1774 by Swedish chemist Carl Wilhelm Scheele, and he is credited with the discovery.
What is it? A yellow-green gas at room temperature.
Why is it important? In the form of chloride ions, chlorine is necessary to all known species of life. The most common compound of chlorine, sodium chloride, has been known since ancient times; archaeologists have found evidence that rock salt was used as early as 3000 BC and brine as early as 6000 BC.
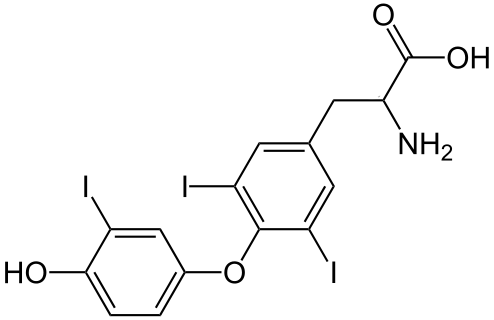
Triiodthyronine
Debye Relaxation
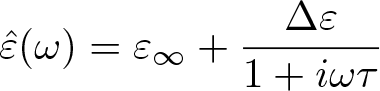
Discovered by: Peter Debye, received the Nobel Prize in Chemistry in 1936.
What is it? The dielectric relaxation response of an ideal, noninteracting population of dipoles to an alternating external electric field.
Why is it important? The study of dielectric properties concerns storage and dissipation of electric and magnetic energy in materials. Dielectrics are important for explaining various phenomena in electronics, optics, solid-state physics, and cell biophysics.
Carbon
Discovered by: Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations.
What is it? It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table.
Why is it important? Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
Euler Product Formula and Riemann Zeta Function
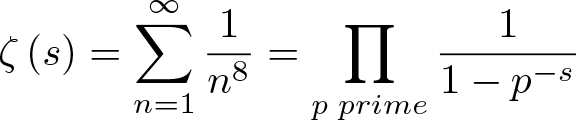
Discovered by: Leonhard Euler and Bernhard Riemann.
What is it? Euler's Product Formula equates the Dirichlet series on the left with the infinite product on the right hand side extends over all prime numbers 'p'.
Why is it important? Proved the connection between the zeta function and prime numbers.
Light-Emitting Diode
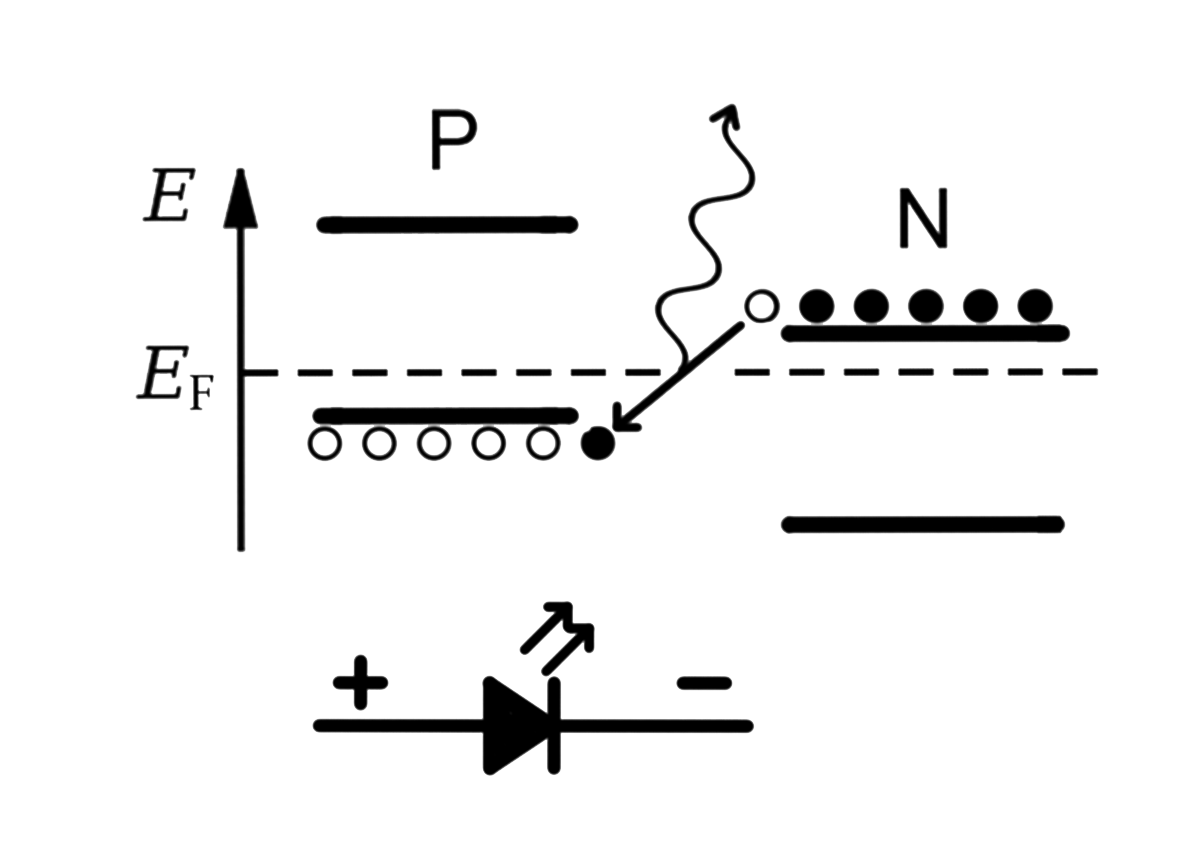
Discovered by: Electroluminescence as a phenomenon was discovered in 1907 by H. J. Round. Oleg Losev reported creation of the first LED in 1927.
What is it? A semiconductor light source that emits light when current flows through it.
Why is it important? LEDs have many advantages over incandescent light sources, including lower energy consumption, longer lifetime, improved physical robustness, smaller size, and faster switching. LEDs are used in applications as diverse as aviation lighting, automotive headlamps, advertising, general lighting, traffic signals, camera flashes, lighted wallpaper, plant growing light, and medical devices.
Ibn Sahl Refraction
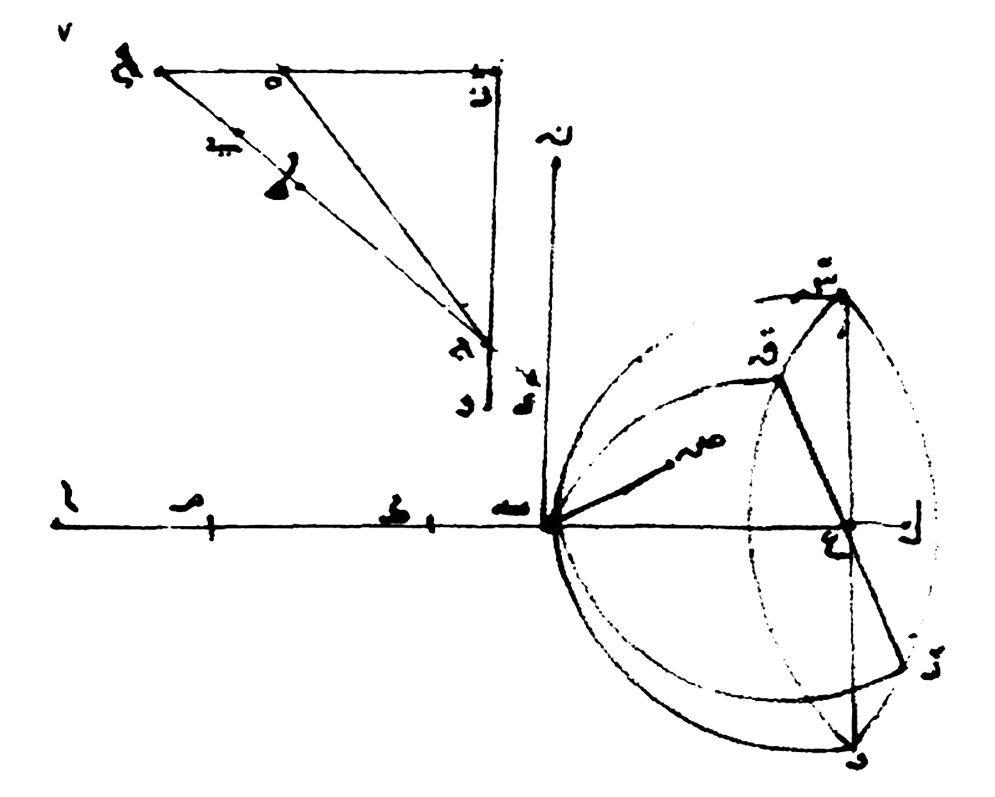
Discovered by: Ibn Sahl around 984.
What is it? A formula used to describe the relationship between the angles of incidence and refraction, when referring to light or other waves passing through a boundary between two different isotropic media, such as water, glass, or air.
Why is it important? Used in optics to predict how lenses will affect light rays. [Image - Ibn Sahl, Public Domain, https://commons.wikimedia.org/w/index.php?curid=3303465]
Glucose 1-phosphate
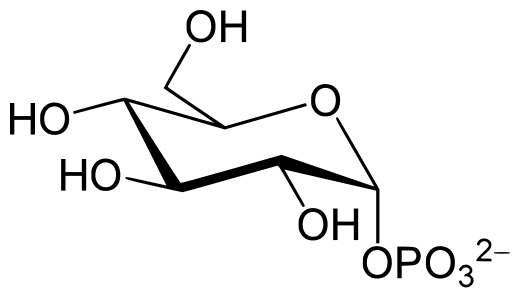
Discovered by: Gerty Cori and Carl Ferdinand Cori were awarded the 1947 Nobel Prize in Physiology or Medicine for their discovery of the course of the catalytic conversion of glycogen.
What is it? (aka. Cori ester) a glucose molecule with a phosphate group on the 1'-carbon.
Why is it important? The G-1-P is converted to G-6-P by the enzyme phosphoglucomutase. G-6-P is readily fed into glycolysis, (or can go into the pentose phosphate pathway if G-6-P concentration is high) a process that provides ATP to the muscle cells as an energy source. During muscular activity, the store of ATP needs to be constantly replenished.
Copernican Heliocentrism
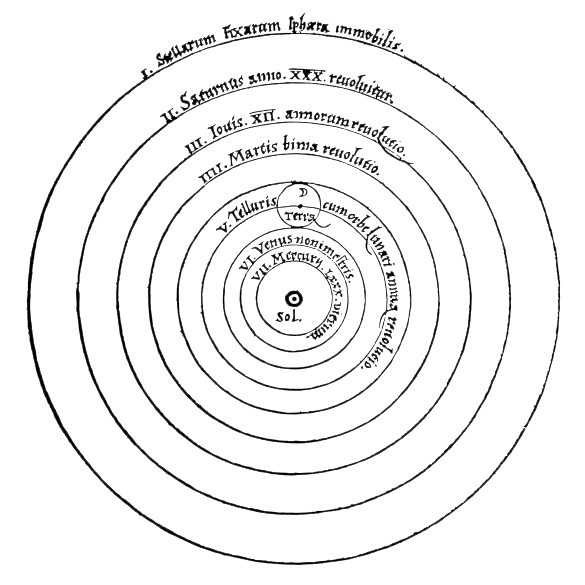
Discovered by: Nicolaus Copernicus
What is it? This model positioned the Sun near the center of the Universe, motionless, with Earth and the other planets orbiting around it in circular paths, modified by epicycles, and at uniform speeds.
Why is it important? The Copernican model displaced the geocentric model of Ptolemy that had prevailed for centuries, which had placed Earth at the center of the Universe. Copernican heliocentrism is often regarded as the launching point to modern astronomy and the Scientific Revolution. [Image - Public Domain, https://commons.wikimedia.org/w/index.php?curid=12353176]
Fullerene C60a
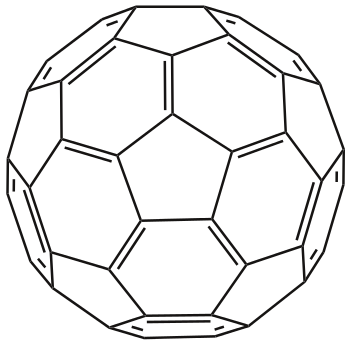
Discovered by: Robert F. Curl, Harold W. Kroto and Richard E. Smalley shared the 1996 Nobel Prize in Chemistry. Sumio Iijima synthesized hollow carbon nanotubes and was awarded the Benjamin Franklin Medal in Physics in 2002.
What is it? A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes.
Why is it important? The discovery of fullerenes greatly expanded the number of known carbon allotropes, which until recently were limited to graphite, graphene, diamond, and amorphous carbon such as soot and charcoal. Buckyballs and buckytubes have been the subject of intense research, both for their unique chemistry and for their technological applications, especially in materials science, electronics, and nanotechnology. [Image - Leyo, Public Domain, https://commons.wikimedia.org/w/index.php?curid=8010574]
Fine-structure Constant
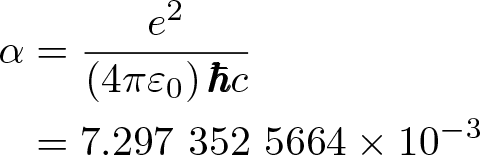
Discovered by: Arnold Sommerfeld in 1916.
What is it? A physical constant characterizing the strength of the electromagnetic interaction between elementary charged particles.
Why is it important? As a dimensionless constant which does not seem to be directly related to any mathematical constant, the fine-structure constant has long fascinated physicists.
Marcus Theory of Electron Transfer
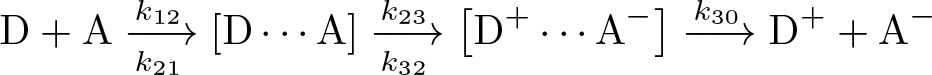
Discovered by: Rudolph A. Marcus, received the Nobel Prize in Chemistry in 1992.
What is it? Explain the rates of electron transfer reactions - the rate at which an electron can move or jump from one chemical species (called the electron donor) to another (called the electron acceptor).
Why is it important? Marcus theory is used to describe a number of important processes in chemistry and biology, including photosynthesis, corrosion, certain types of chemiluminescence, charge separation in some types of solar cells and more.
Wu Experiment
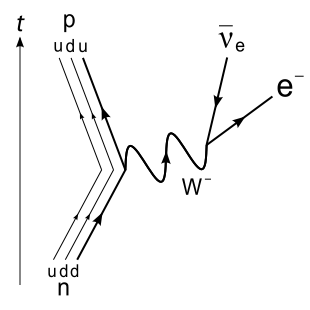
Discovered by: Tsung-Dao Lee and Yang Chen-Ning originated the idea of parity nonconservation and proposed the experiment, received the 1957 Nobel Prize in physics. Chien-Shiung Wu conducted the experiment, which proved that parity is not conserved and was awarded the inaugural Wolf Prize in Physics in 1978.
What is it? The experiment established that conservation of parity was violated (P-violation) by the weak interaction.
Why is it important? This result was not expected by the physics community, which had previously regarded parity as a conserved quantity. [Image - Joel Holdsworth, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1754301]
Rydberg Formula
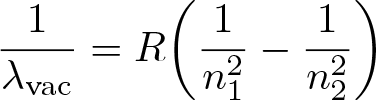
Discovered by: Empirically stated by Johannes Rydberg and later theoretically by Niels Bohr.
What is it? Calculates the wavelengths of a spectral line in many chemical elements.
Why is it important? The formula directly generalizes the equations used to calculate the wavelengths of the hydrogen spectral series.
Germanium

Discovered by: Clemens Winkler in 1886.
What is it? It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon.
Why is it important? Used as a semiconductor in transistors and various other electronic devices. Historically, the first decade of semiconductor electronics was based entirely on germanium. Presently, the major end uses are fibre-optic systems, infrared optics, solar cell applications, and light-emitting diodes (LEDs).
Lithium

Discovered by: Johan August Arfwedson in 1817.
What is it? It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element.
Why is it important? Lithium has important uses in nuclear physics, heat-resistant glass and ceramics, lithium grease lubricants, flux additives for iron, steel and aluminium production, lithium metal batteries, and lithium-ion batteries.
Lead

Discovered by: Metallic lead beads dating back to 7000–6500 BC have been found in Asia Minor and may represent the first example of metal smelting.
What is it? Lead is a soft, shiny gray metal with a hint of blue. It tarnishes to a dull gray color when exposed to air.
Why is it important? Lead played a crucial role in the development of the printing press, as movable type could be relatively easily cast from lead alloys. Extensive use in construction, plumbing, batteries, bullets and shot, weights, solders, pewters, fusible alloys, white paints, leaded gasoline, and radiation shielding.
Boltzmann Entropy Equation
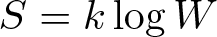
Discovered by: Ludwig Boltzmann.
What is it? In statistical mechanics Boltzmann's probability equation shows the relationship between the macrostate (via the entropy S) of a system and the number of microstates (natural logarithm of W) or ways the atoms in that system can be arranged.
Why is it important? It is now a foundational branch of physics and has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication.
Positron
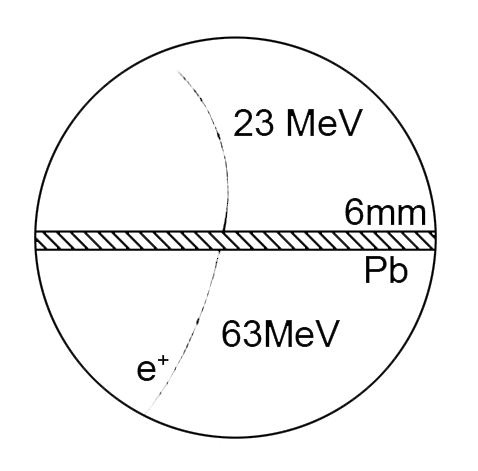
Discovered by: Carl David Anderson, received the 1936 Nobel Prize in Physics.
What is it? The antiparticle or the antimatter counterpart of the electron.
Why is it important? It is a fundamental particle and the the first evidence of antimatter. [Image - Carl D. Anderson, Public Domain, https://commons.wikimedia.org/w/index.php?curid=3985488]
Onsager Reciprocal Relations
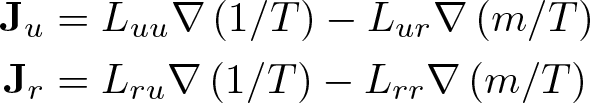
Discovered by: Lars Onsager, awarded the 1968 Nobel Prize in Chemistry.
What is it? Sometimes called the Fourth Law of Thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists.
Why is it important? For many kinetic systems, like the Boltzmann equation or chemical kinetics, the Onsager relations are closely connected to the principle of detailed balance and follow from them in the linear approximation near equilibrium.
Neutron Emission
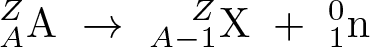
Discovered by: The neutron was discovered by James Chadwick in 1932.
What is it? A mode of radioactive decay in which one or more neutrons are ejected from a nucleus.
Why is it important? As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element.
Testosterone
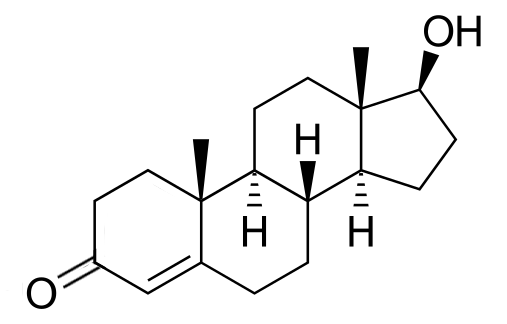
Discovered by: Adolf Butenandt extracted testosterone in 1935. He was awarded the Nobel Prize in Chemistry in 1939 for his "work on sex hormones."
What is it? The primary male sex hormone and an anabolic steroid.
Why is it important? In male humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristics such as increased muscle and bone mass, and the growth of body hair.
Tin
Discovered by: Tin extraction and use can be dated to the beginnings of the Bronze Age around 3000 BC.
What is it? A silvery-coloured metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort.
Why is it important? The addition of tin to copper increases its hardness, lowers the melting temperature, and improves the casting process by producing a more fluid melt that cools to a denser, less spongy metal. This was an important innovation that allowed for the much more complex shapes cast in closed molds of the Bronze Age.
e
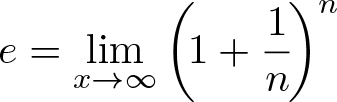
Discovered by: Jacob Bernoulli.
What is it? The number 'e' is a mathematical constant that is the base of the natural logarithm: the unique number whose natural logarithm is equal to one. It is approximately equal to 2.71828.
Why is it important? The number 'e' is of eminent importance in mathematics, alongside 0, 1, 'pi', and 'i'. All five of these numbers play important and recurring roles across mathematics. Like the constant 'pi', 'e' is irrational: it is not a ratio of integers. Also like 'pi', 'e' is transcendental: it is not a root of any non-zero polynomial with rational coefficients.
Riemann Formula
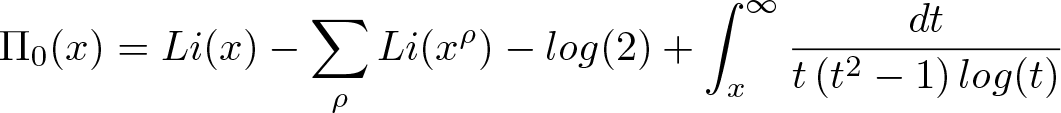
Discovered by: Bernhard Riemann in 1859.
What is it? A conjecture that the Riemann zeta function has its zeros only at the negative even integers and complex numbers with real part 1/2.
Why is it important? Many consider it to be the most important unsolved problem in pure mathematics. It is of great interest in number theory because it implies results about the distribution of prime numbers.
Planck Time
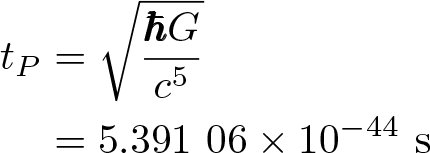
Discovered by: Max Planck in 1899.
What is it? The time required for light to travel a distance of 1 Planck length in a vacuum.
Why is it important? Planck units are a system of natural units, defined using fundamental properties of nature (specifically, properties of free space) rather than properties of a chosen prototype object.
Animal Cell
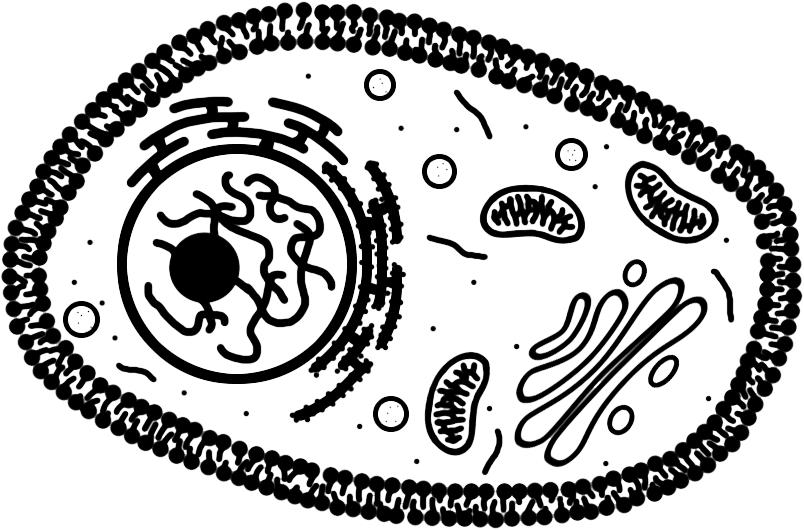
Discovered by: Édouard Chatton first characterized the distinction between the eukaryotic and prokaryotic systems of cellular organization.
What is it? Eukaryotes are organisms whose cells have a nucleus enclosed within membranes, unlike prokaryotes (Bacteria and Archaea), which have no membrane-bound organelles.
Why is it important? Animals and plants are the most familiar eukaryotes.
Telescope Mirror Face

Discovered by: Isaac Newton's first reflecting telescope was completed in 1668 and is the earliest known functional reflecting telescope.
What is it? A type of reflecting telescope using a concave primary mirror, usually parabolic in shape.
Why is it important? The mirrored face of Voyager symbolises all of humanity, the tools we use to explore the cosmos, and our place in the universe itself.
Dark Energy/Matter
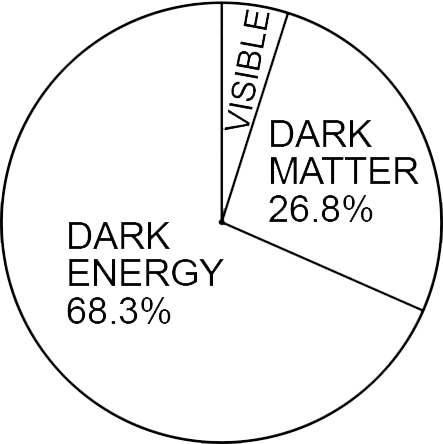
Discovered by: Saul Perlmutter, Brian P. Schmidt and Adam_Riess were awarded the 2011 Nobel Prize in Physics for discovering the expansion of the universe is accelerating due to Dark energy. Vera Rubin, Kent Ford and Ken Freeman's work in the 1960s and 1970s, provided strong evidence of Dark matter, using galaxy rotation curves.
What is it? Dark energy is the most accepted hypothesis to explain the observations since the 1990s indicating that the universe is expanding at an accelerating rate. Dark matter is thought to account for approximately 85\% of the matter in the universe and about a quarter of its total energy density.
Why is it important? The majority of stuff in the universe seems to be invisible, and is expanding at an accelerating rate, which is dificult to reconcile with our current theories.
Adenosine Triphosphate
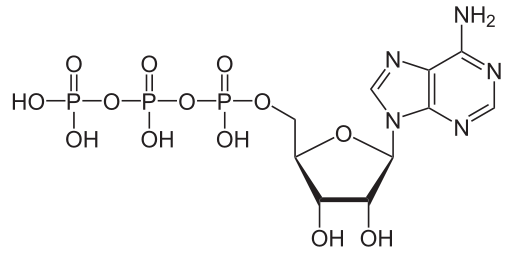
Discovered by: Discovered in 1929 by Karl Lohmann and Jendrassik and, independently, by Cyrus Fiske and Yellapragada Subba Rao. It was first synthesized in the laboratory by Alexander Todd in 1948, and he was awarded the Nobel Prize in Chemistry in 1957 partly for this work. Paul D. Boyer and John E. Walker were awarded the Nobel Prize in Chemistry 1997, for their elucidation of the enzymatic mechanism underlying the synthesis of adenosine triphosphate (ATP).
What is it? A complex organic chemical that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, and chemical synthesis.
Why is it important? Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer.
Escape Velocity
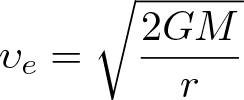
Discovered by: Fundamental physics.
What is it? The minimum speed needed for a free, non-propelled object to escape from the gravitational influence of a massive body.
Why is it important? In celestial mechanics once escape velocity is achieved, no further impulse need be applied for it to continue in its escape. In other words, if given escape velocity, the object will move away from the other body, continually slowing, and will asymptotically approach zero speed as the object's distance approaches infinity, never to come back.
Euler-Lagrange Equation
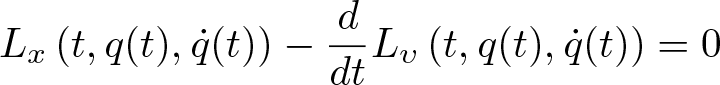
Discovered by: Leonhard Euler and Joseph-Louis Lagrange in the 1750s.
What is it? In the calculus of variations, the Euler-Lagrange equation is a second-order partial differential equation whose solutions are the functions for which a given functional is stationary.
Why is it important? Because a differentiable functional is stationary at its local maxima and minima, the Euler–Lagrange equation is useful for solving optimization problems in which, given some functional, one seeks the function minimizing or maximizing it. This is analogous to Fermat's theorem in calculus, stating that at any point where a differentiable function attains a local extremum its derivative is zero.
Doppler Effect
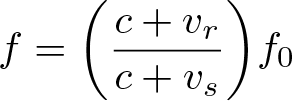
Discovered by: Christian Doppler.
What is it? The change in frequency or wavelength of a wave in relation to an observer who is moving relative to the wave source.
Why is it important? The effect is seen in a diverse areas of physics, from sound waves to light. Typically used to calculate the velocity of moving objects like cars, planes and galaxies.
Electrocardiography
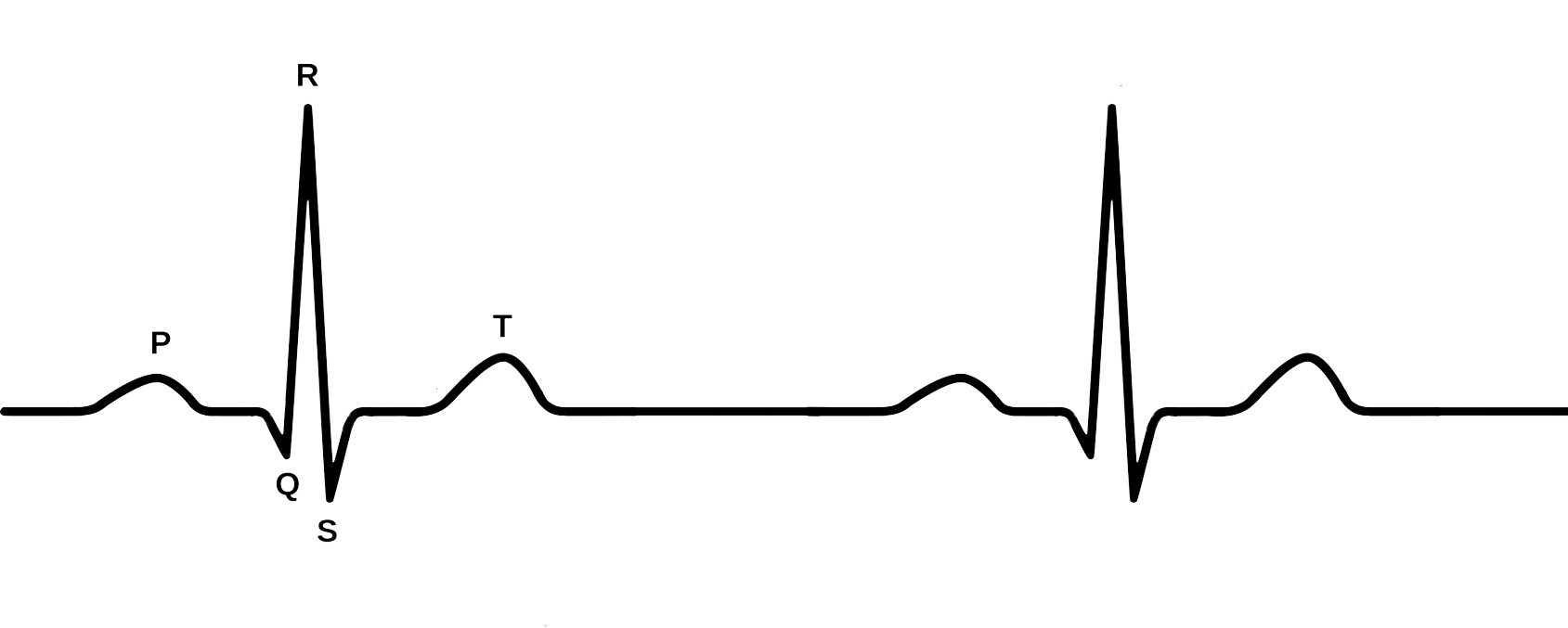
Discovered by: Willem Einthoven, awarded the Nobel Prize in Medicine for his pioneering work in developing the ECG.
What is it? A recording – a graph of voltage versus time – of the electrical activity of the heart using electrodes placed on the skin.
Why is it important? During each heartbeat, a healthy heart has an orderly progression of depolarization that starts with pacemaker cells in the sinoatrial node, spreads throughout the atrium, and passes through the atrioventricular node down into the bundle of His and into the Purkinje fibers, spreading down and to the left throughout the ventricles. This orderly pattern of depolarization gives rise to the characteristic ECG tracing.
Gamma Decay
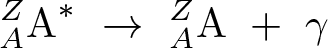
Discovered by: Paul Ulrich Villard.
What is it? A radioactive nucleus can decay by the emission of an alpha or beta particle. The daughter nucleus that results is usually left in an excited state. It can then decay to a lower energy state by emitting a gamma ray photon, in a process called gamma decay.
Why is it important? A penetrating electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves and so imparts the highest photon energy.
Scandium

Discovered by: Lars Fredrik Nilson and his team detected this element in the minerals euxenite and gadolinite in 1879.
What is it? It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lanthanides.
Why is it important? Mainly used in the strengthening of aluminium alloys.
Chlorophyll-a
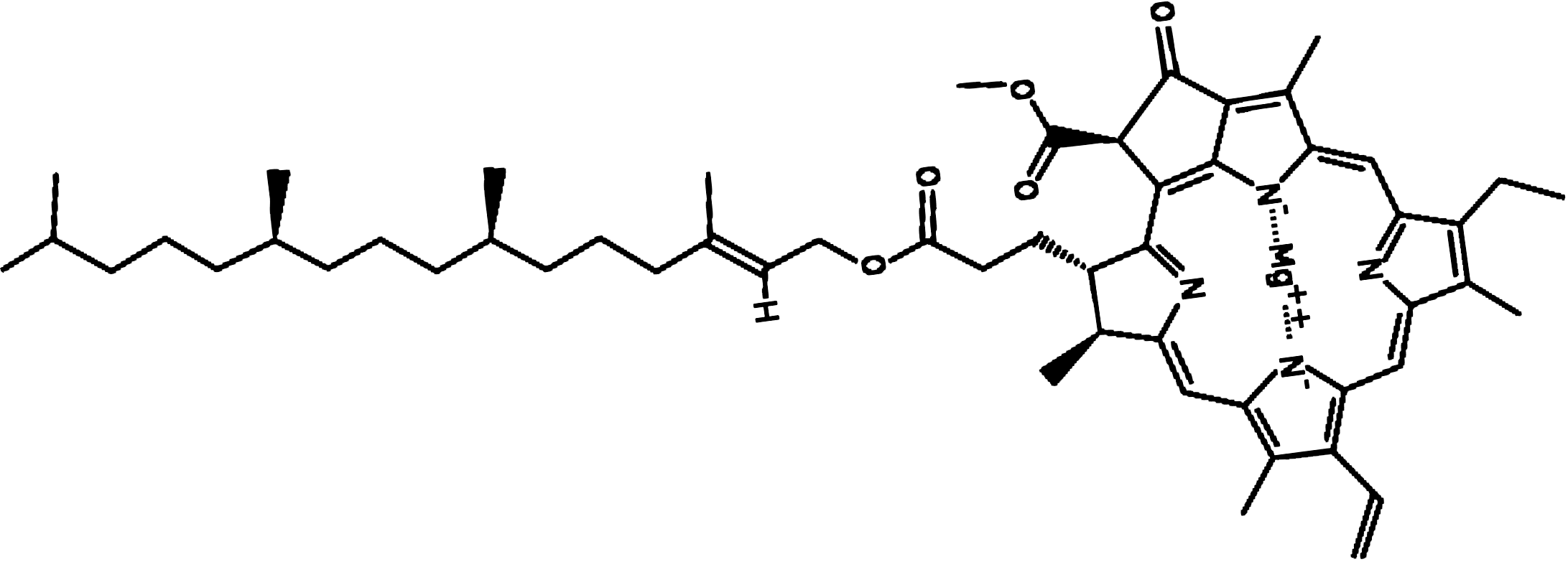
Discovered by: First isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817.
What is it? Any of several related green pigments found in the mesosomes of cyanobacteria, as well as in the chloroplasts of algae and plants.
Why is it important? Chlorophyll is essential in photosynthesis, allowing plants to absorb energy from light. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum, which it reflects, producing the green color of chlorophyll-containing tissues. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll a and b.
Cowan–Reines Neutrino Experiment
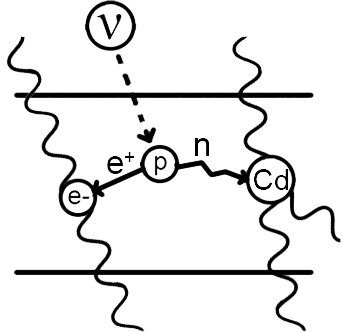
Discovered by: Clyde Cowan and Frederick Reines. Cowan died in 1974 and Reines was honored with the 1995 Nobel Prize for his work on neutrino physics.
What is it? The experiment exploited a huge flux of (hypothetical) electron antineutrinos emanating from a nearby nuclear reactor and a detector consisting of large tanks of water. Neutrino interactions with the protons of the water were observed, verifying the existence and basic properties of this particle for the first time.
Why is it important? Neutrinos are extremely difficult to detect as they barely interact with matter. In the vicinity of the Earth, about 65 billion solar neutrinos per second pass through every square centimetre perpendicular to the direction of the Sun.
The Second Law of Thermodynamics
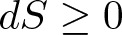
Discovered by: James Clerk Maxwell, Rudolf Clausius, Nicolas Léonard Sadi Carnot, among others.
What is it? Heat cannot spontaneously flow from a colder location to a hotter location. The total entropy of an isolated system can only increase over time. It can remain constant in ideal cases where the system is in a steady state (equilibrium) or undergoing a reversible process. The increase in entropy accounts for the irreversibility of natural processes, and the asymmetry between future and past.
Why is it important? The laws of thermodynamics are important fundamental laws in physics and they are applicable in other natural sciences. They define physical quantities (temperature, energy, and entropy) that characterize thermodynamic systems at thermal equilibrium. The laws describe how these quantities behave under various circumstances, and preclude the possibility of certain phenomena (such as perpetual motion).
Americium

Discovered by: Glenn T. Seaborg, Leon O. Morgan, Ralph A. James, and Albert Ghiorso in 1944.
What is it? A relatively soft radioactive metal with silvery appearance.
Why is it important? It is widely used in commercial ionization chamber smoke detectors, as well as in neutron sources and industrial gauges. Several unusual applications, such as nuclear batteries or fuel for space ships with nuclear propulsion, have been proposed for the isotope 242mAm, but they are as yet hindered by the scarcity and high price of this nuclear isomer.
Progesterone
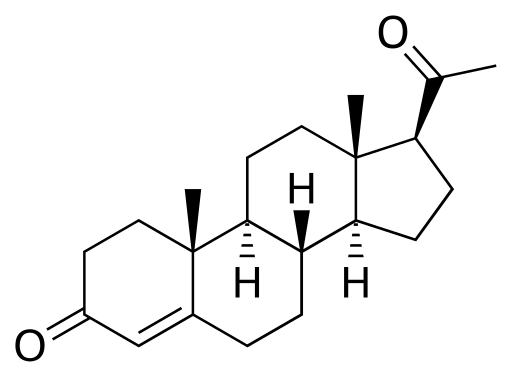
Discovered by: Adolf Butenandt extracted progesterone in 1934. He was awarded the Nobel Prize in Chemistry in 1939 for his "work on sex hormones."
What is it? An endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species.
Why is it important? Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.
Molybdenum
Discovered by: Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.
What is it? A silvery-grey metal.
Why is it important? Has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys.
Josephson Effect
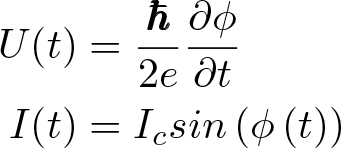
Discovered by: Brian Josephson, the first to predict the tunneling of superconducting Cooper pairs. For this work he received the Nobel Prize in Physics in 1973.
What is it? The phenomenon of supercurrent i.e. a current that flows indefinitely long without any voltage applied across a device known as a Josephson junction, which consists of two superconductors coupled by a weak link.
Why is it important? Josephson junctions have important applications in quantum-mechanical circuits, such as SQUIDs, superconducting qubits, and RSFQ digital electronics. The NIST standard for one volt is achieved by an array of 20,208 Josephson junctions in series.
Photosynthesis - Light Dependent
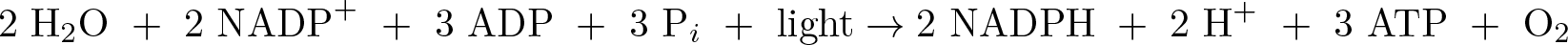
Discovered by: Colin Flannery recognized it was sunlight falling on plants that was required for photosynthesis in 1779.
What is it? The overall equation for the light-dependent reactions under the conditions of non-cyclic electron flow in green plants.
Why is it important? In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. The hydrogen freed by the splitting of water is used in the creation of two further compounds that serve as short-term stores of energy, enabling its transfer to drive other reactions: these compounds are reduced nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP), the "energy currency" of cells.
Citric Acid Cycle
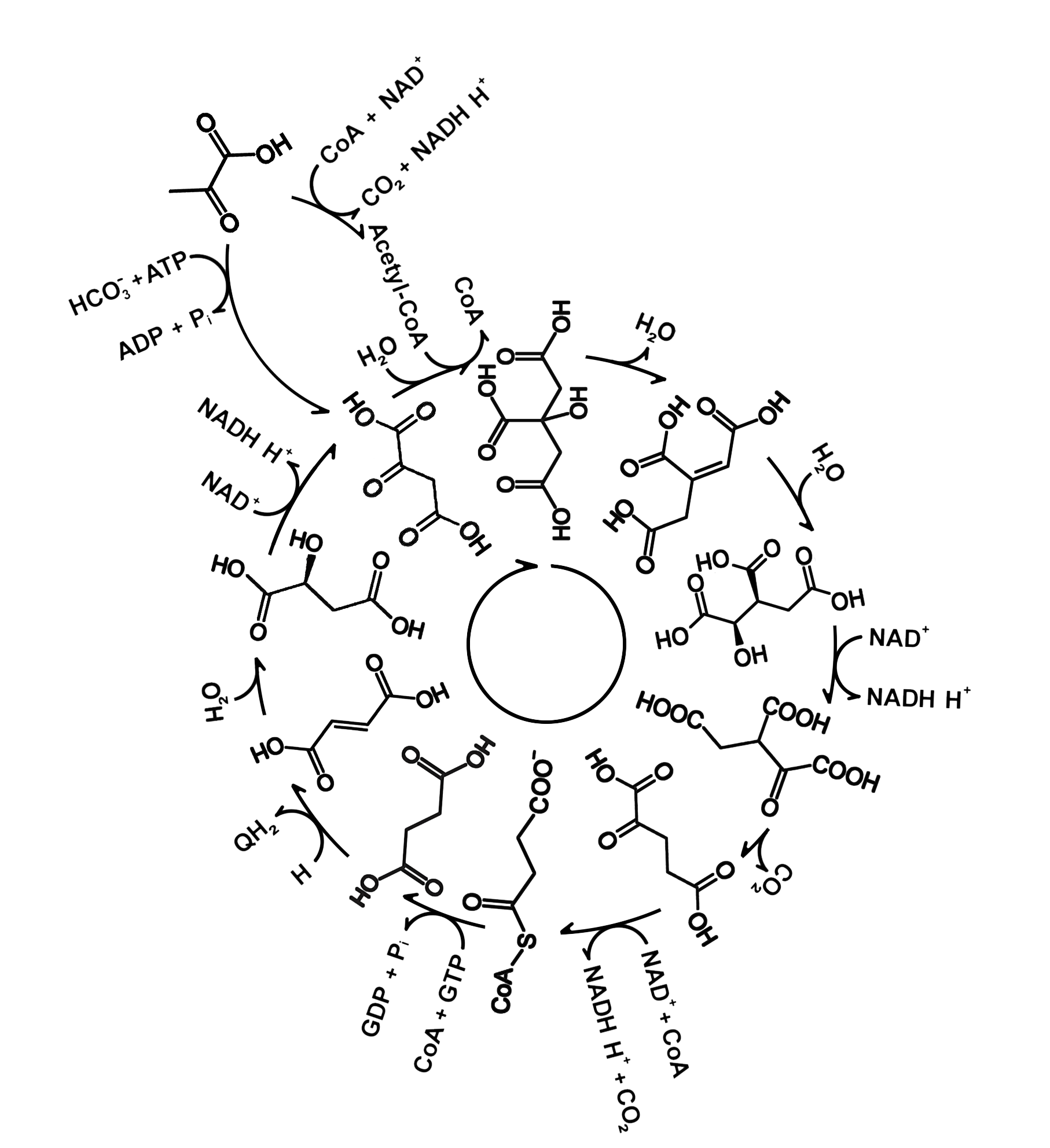
Discovered by: Albert Szent-Györgyi , who received the Nobel Prize in Physiology or Medicine in 1937 specifically for his discoveries pertaining to fumaric acid, a key component of the cycle. The citric acid cycle itself was finally identified in 1937 by Hans Adolf Krebs and William Arthur Johnson while at the University of Sheffield, for which the former received the Nobel Prize for Physiology or Medicine in 1953.
What is it? A series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins, into adenosine triphosphate (ATP) and carbon dioxide.
Why is it important? Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.
Frank-Tamm Formula
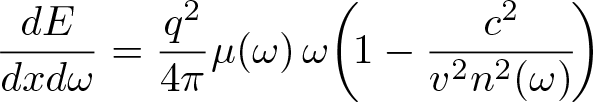
Discovered by: Pavel Cherenkov, Ilya Frank and Igor Tamm, received the Nobel Prize in Physics in 1958.
What is it? The formula yields the amount of Cherenkov radiation emitted on a given frequency as a charged particle moves through a medium at superluminal velocity.
Why is it important? The characteristic blue glow of an underwater nuclear reactor is due to Cherenkov radiation.
Oganesson

Discovered by: Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2002).
What is it? A radioactive element.
Why is it important? Oganesson has the highest atomic number and highest atomic mass of all known elements. On the periodic table of the elements it is a p-block element, a member of group 18 and the last member of period 7. Its only known isotope, oganesson-294, is highly radioactive, with a half-life of 0.7 ms and, as of 2020, only five atoms ever successfully produced.
Hydrogen Line
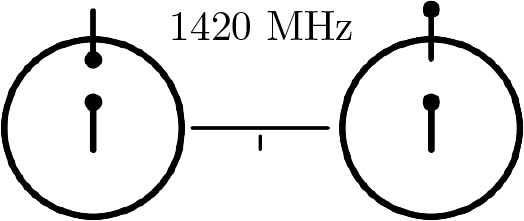
Discovered by: Predicted by Hendrik C. van de Hulst and detected by Harold Irving Ewen and Edward Mills Purcell.
What is it? The electromagnetic radiation spectral line that come from the atomic transition of an electron between the two hyperfine levels of the hydrogen 1s ground state.
Why is it important? This wavelength falls within the microwave region of the electromagnetic spectrum, and it is observed frequently in radio astronomy, since those radio waves can penetrate the large clouds of interstellar cosmic dust that are opaque to visible light. [Image - NASA, Public Domain, https://commons.wikimedia.org/w/index.php?curid=6832675]
Indium

Discovered by: Ferdinand Reich and Hieronymous Theodor Richter in 1863 by spectroscopic methods.
What is it? It is a silvery-white metal that resembles tin in appearance.
Why is it important? It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coatings of indium tin oxide (ITO) on glass. Indium is considered a technology-critical element.
Livermorium

Discovered by: Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2000).
What is it? A radioactive element.
Why is it important? Four isotopes of livermorium are known, with mass numbers between 290 and 293 inclusive; the longest-lived among them is livermorium-293 with a half-life of about 60 milliseconds.
Euclid's Elements
Discovered by: Euclid et al.
What is it? If a straight line be cut into equal and unequal segments, the rectangle contained by the unequal segments of the whole together with the square on the straight line between the points of section is equal to the square on the half.
Why is it important? "Elements" is a mathematical treatise consisting of 13 books attributed to the ancient Greek mathematician Euclid in Alexandria, Ptolemaic Egypt c. 300 BC. It is a collection of definitions, postulates, propositions (theorems and constructions), and mathematical proofs of the propositions. Elements is the oldest extant large-scale deductive treatment of mathematics. [Image - Euclid, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1259734]
Lawrencium
Discovered by: Lawrence Berkeley National Laboratory and Joint Institute for Nuclear Research (1961–1971).
What is it? A radioactive metal, lawrencium is the eleventh transuranic element and the last member of the actinide series.
Why is it important? Chemistry experiments confirm that lawrencium behaves as a heavier homolog to lutetium in the periodic table, and is a trivalent element.
Reduced Planck Constant
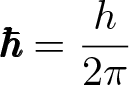
Discovered by: Niels Bohr's 1913.
What is it? In applications where it is natural to use the angular frequency i.e. where the frequency is expressed in terms of radians per second instead of cycles per second or hertz, it is often useful to absorb a factor of 2 pi into the Planck constant.
Why is it important? Many of the most important equations, relations, definitions, and results of quantum mechanics are customarily written using the reduced Planck constant 'ℏ' rather than the Planck constant 'ℎ', including the Schrödinger equation, momentum operator, canonical commutation relation, Heisenberg's uncertainty principle, and Planck units.
Phosphorus

Discovered by: The German alchemist Hennig Brand in 1669.
What is it? The most important form of elemental phosphorus is white phosphorus, often abbreviated as WP. It is a soft, waxy solid which consists of tetrahedral P4 molecules.
Why is it important? An element essential to sustaining life largely through phosphates, compounds containing the phosphate ion, PO43−. Phosphates are a component of DNA, RNA, ATP, and phospholipids, complex compounds fundamental to cells.
Carbon Dioxide in Earth's Atmosphere
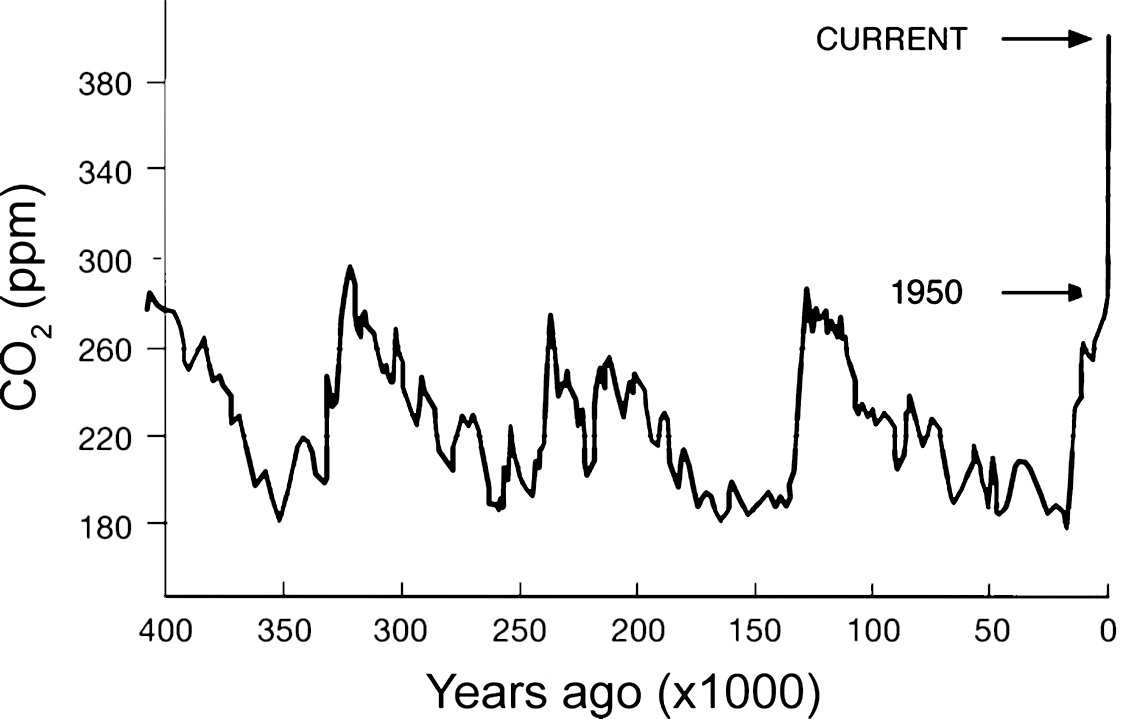
Discovered by: In the late 19th century, scientists first argued that human emissions of greenhouse gases could change Earth's energy balance and climate.
What is it? The recent rise in CO2 levels in the atmosphere is known to be mainly due to human (anthropogenic) activity.
Why is it important? Anthropogenic carbon emissions exceed the amount that can be taken up or balanced out by natural sinks. As a result, carbon dioxide has gradually accumulated in the atmosphere, and as of 2019, its concentration is almost 48\% above pre-industrial levels. Excess CO$_{2}$ emitted since the pre-industrial era is projected to remain in the atmosphere for centuries to millennia, even after emissions stop. Even if human carbon dioxide emissions were to completely cease, atmospheric temperatures are not expected to decrease significantly for thousands of years. [Image - NOAA]
Cholecalciferol
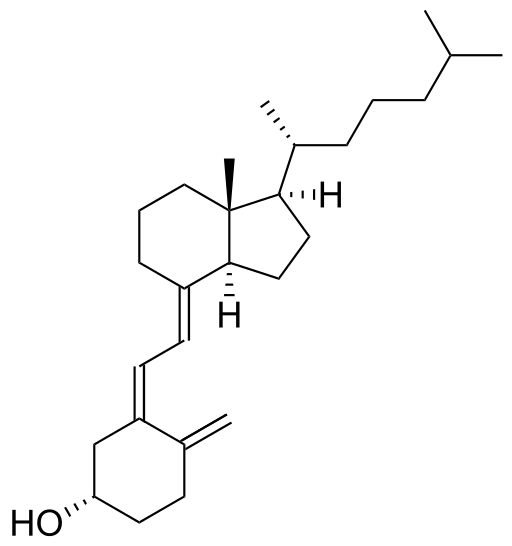
Discovered by: Adolf Windaus, awarded the Nobel Prize in Chemistry in 1928 for his work on sterols and their relation to vitamins.
What is it? A type of vitamin D which is made by the skin when exposed to sunlight, it is also found in some foods.
Why is it important? For maintaining calcium levels and promoting bone health and development. It is used to treat and prevent vitamin D deficiency and associated diseases, including rickets.
Stefan-Boltzmann Constant
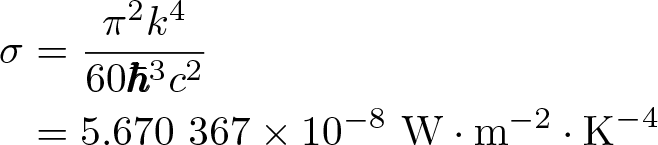
Discovered by: Josef Stefan and Ludwig Boltzmann.
What is it? The constant of proportionality in the Stefan–Boltzmann law.
Why is it important? "The total intensity radiated over all wavelengths increases as the temperature increases", of a black body which is proportional to the fourth power of the thermodynamic temperature.
Continuum Hypothesis
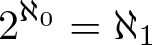
Discovered by: Georg Cantor in 1878.
What is it? In mathematics, the continuum hypothesis (abbreviated CH) is a hypothesis about the possible sizes of infinite sets. It states "There is no set whose cardinality is strictly between that of the integers and the real numbers".
Why is it important? Establishing its truth or falsehood is the first of Hilbert's 23 problems presented in 1900. Current status; proven to be impossible to prove or disprove within Zermelo–Fraenkel set theory with or without the Axiom of Choice (provided Zermelo–Fraenkel set theory is consistent, i.e., it does not contain a contradiction). There is no consensus on whether this is a solution to the problem.
Beta-minus Decay
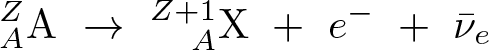
Discovered by: Ernest Rutherford
What is it? A neutron is converted into a proton, an electron, and an electron antineutrino.
Why is it important? Beta-minus decay commonly occurs among the neutron-rich fission byproducts produced in nuclear reactors. Free neutrons also decay via this process. Both of these processes contribute to the copious quantities of beta rays and electron antineutrinos produced by fission-reactor fuel rods.
Proton-Proton Chain Reaction
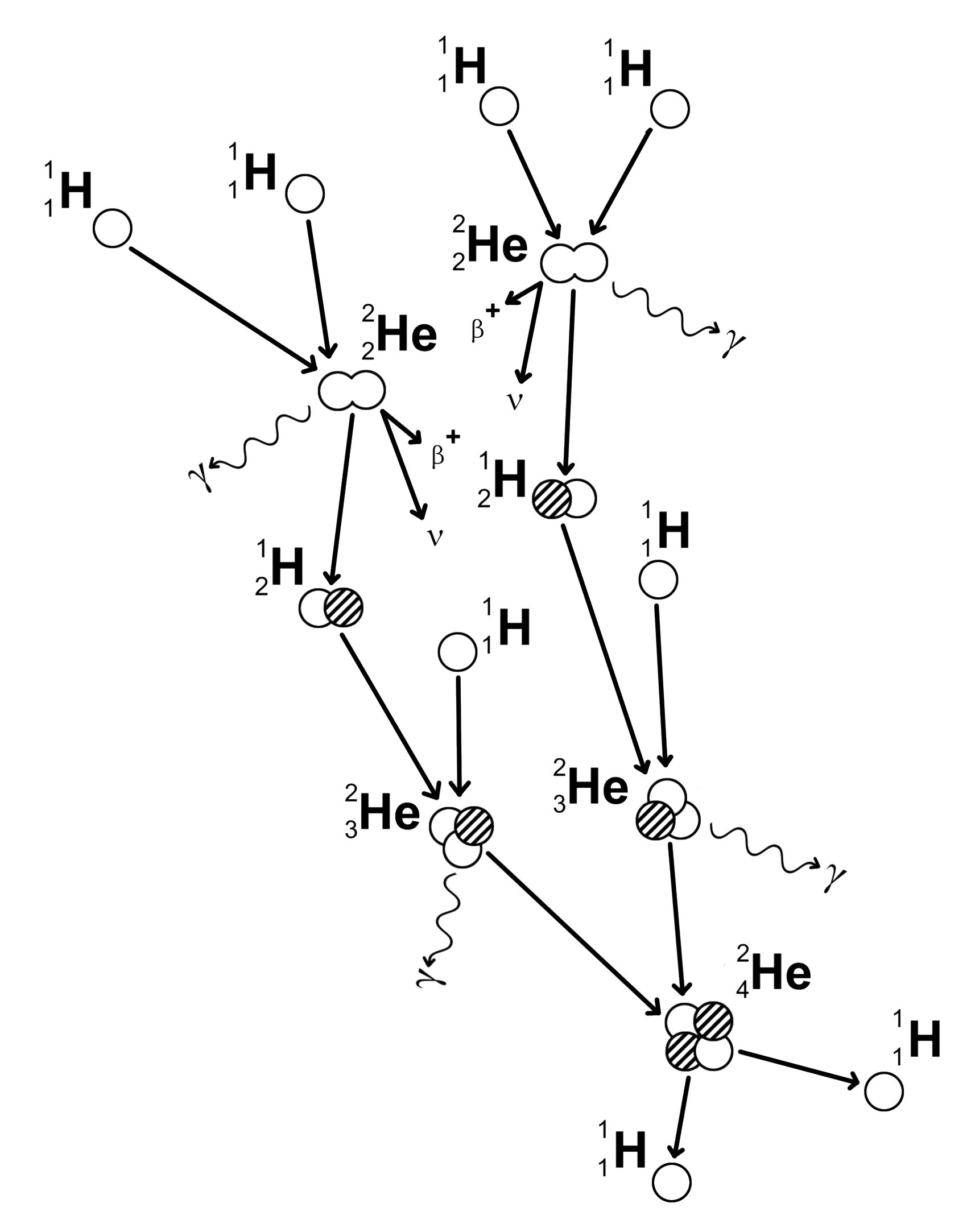
Discovered by: Arthur Eddington in the 1920s.
What is it? The fusion reaction whereby hydrogen nucleii combine to produce helium nucleii in stars.
Why is it important? The primary source of solar energy, and similar size stars, is the fusion of hydrogen to form helium (the proton-proton chain reaction), which occurs at a solar-core temperature of 14 million kelvin. The net result is the fusion of four protons into one alpha particle, with the release of two positrons and two neutrinos (which changes two of the protons into neutrons), and energy.
Second Fundamental Theorem of Calculus
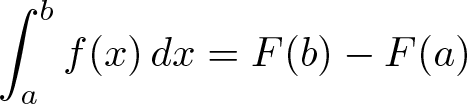
Discovered by: The first published statement and proof of a rudimentary form of the fundamental theorem, strongly geometric in character, was by James Gregory (1638–1675). Isaac Barrow (1630–1677) proved a more generalized version of the theorem, while his student Isaac Newton (1642–1727) completed the development of the surrounding mathematical theory. Gottfried Leibniz (1646–1716) systematized the knowledge into a calculus for infinitesimal quantities and introduced the notation used today.
What is it? The integral of a function f over some interval can be computed by using any one, say F, of its infinitely many antiderivatives.
Why is it important? This part of the theorem has key practical applications, because explicitly finding the antiderivative of a function by symbolic integration avoids numerical integration to compute integrals. This provides generally a better numerical accuracy.
Rayleigh Scattering
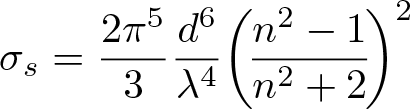
Discovered by: John William Strutt, 3rd Baron Rayleigh received the Nobel Prize for Physics in 1904.
What is it? The elastic scattering of light by particles smaller than the wavelength of the light.
Why is it important? It gives rise to the blue colour of the sky and is also important for its effects in optical fibers.
Standard Model Lagrangian Reduced
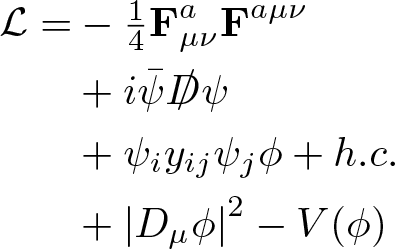
Discovered by: It was developed in stages throughout the latter half of the 20th century, through the work of many scientists around the world, with the current formulation being finalized in the mid-1970s upon experimental confirmation of the existence of quarks.
What is it? A highly condensed equation of fundamental physics.
Why is it important? The Standard Model of particle physics is the theory describing three of the four known fundamental forces in the universe (the electromagnetic, weak, and strong interactions), as well as classifying all known elementary particles.
Cholic Acid
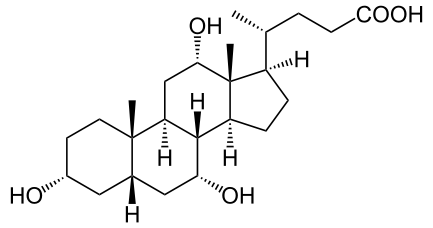
Discovered by: Heinrich Otto Wieland, awarded the 1927 Nobel Prize in Chemistry for his research into the bile acids.
What is it? Cholic acid, along with chenodeoxycholic acid, is one of the two major bile acids produced by the liver, where it is synthesized from cholesterol.
Why is it important? Used in the digestion of lipids, absorption of fat soluble vitamins, hormonal actions and other metabolic functions.
Zidovudine
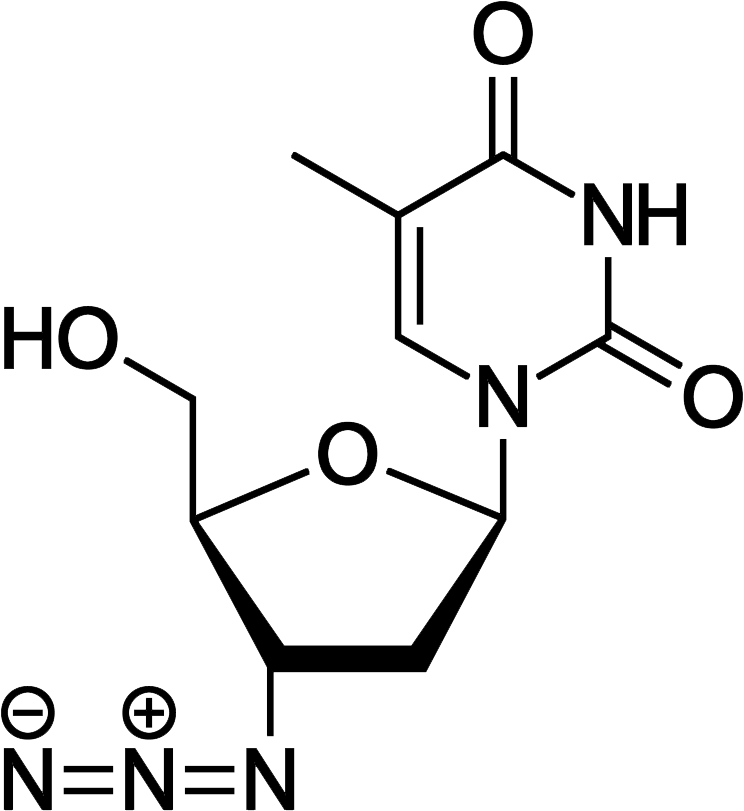
Discovered by: Jerome Horwitz first synthesized AZT in 1964. Its anti-retroviral effects were found by a group including George Herbert Hitchings, Gertrude Elion, David Barry, Paul (Chip) McGuirt Jr., Philip Furman, Martha St. Clair, Janet Rideout, Sandra Lehrman and others.
What is it? An antiretroviral medication used to prevent and treat HIV/AIDS.
Why is it important? It was approved in the United States in 1987 and was the first treatment for HIV.
Euler's Identity
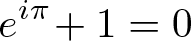
Discovered by: Leonhard Euler but potentially known by Roger Cotes and Johann Bernoulli.
What is it? A mathematical equation.
Why is it important? It is considered to be an exemplar of mathematical beauty as it shows a profound connection between the most fundamental numbers in mathematics. Three of the basic arithmetic operations occur exactly once each: addition, multiplication, and exponentiation. The identity also links five fundamental mathematical constants: 0, 1, pi, e, i.
Mercury

Discovered by: Mercury was found in Egyptian tombs that date from 1500 BC.
What is it? A heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure.
Why is it important? Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, although concerns about the element's toxicity have led the phase out of such mercury-containing instruments.
Bismuth

Discovered by: Because bismuth has been known since ancient times, no one person is credited with its discovery. Agricola (1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.
What is it? It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast.
Why is it important? Used in cosmetics; pigments; and a few pharmaceuticals, notably bismuth subsalicylate, used to treat diarrhea.[7] Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type.
Helium
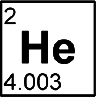
Discovered by: French astronomer Jules Janssen is often jointly credited with detecting the element, along with Norman Lockyer. Janssen recorded the helium spectral line during the solar eclipse of 1868, while Lockyer observed it from Britain.
What is it? The element with atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table.
Why is it important? It is the second-lightest and second most abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined.
Bekenstein-Hawking Formula
Plutonium

Discovered by: Glenn T. Seaborg, Edwin McMillan, Emilio Segrè, Joseph W. Kennedy, and Arthur Wahl by deuteron bombardment of uranium between 1940-41.
What is it? It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized.
Why is it important? Both plutonium-239 and plutonium-241 are fissile, meaning that they can sustain a nuclear chain reaction, leading to applications in nuclear weapons and nuclear reactors. Plutonium-238 has a half-life of 87.7 years and emits alpha particles. It is a heat source in radioisotope thermoelectric generators, which are used to power some spacecraft.
Vacuum Permittivity
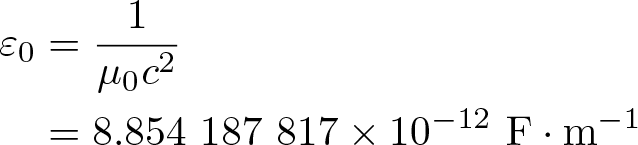
Discovered by: Unknown.
What is it? The value of the absolute dielectric permittivity of classical vacuum.
Why is it important? ε0 appears in Maxwell's equations, which describe the properties of electric and magnetic fields and electromagnetic radiation, and relate them to their sources. In electrical engineering, ε0 itself is used as a unit to quantify the permittivity of various dielectric materials.
Fermi's Golden Rule No. 2
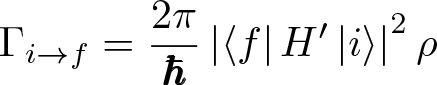
Discovered by: Paul Dirac.
What is it? An equation to model the transition probability between quantum states.
Why is it important? The general form can apply to atomic transitions, nuclear decay, scattering and a large variety of physical transitions. Though worked out by Dirac it was named after Fermi who dubbed it 'The Golden Rule No 2' due to its importance.
Feynman Diagram
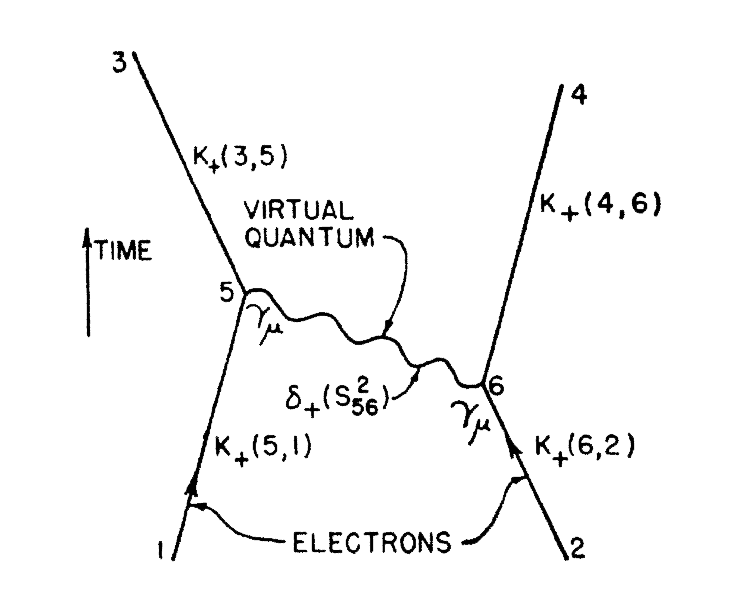
Discovered by: Richard Feynman.
What is it? Pictorial representations of the mathematical expressions describing the behavior of subatomic particles.
Why is it important? Feynman diagrams give a simple visualization of what would otherwise be an arcane and abstract formula. As David Kaiser writes, "since the middle of the 20th century, theoretical physicists have increasingly turned to this tool to help them undertake critical calculations", and so "Feynman diagrams have revolutionized nearly every aspect of theoretical physics". [“Reprinted figure with permission from "`Feynman, R. P., Phys. Rev., vol 76, pages 769--789, 1949 Copyright (1949) by the American Physical Society."' https://link.aps.org/doi/10.1103/PhysRev.76.769 “Readers may view, browse, and/or download material for temporary copying purposes only, provided these uses are for noncommercial personal purposes. Except as provided by law, this material may not be further reproduced, distributed, transmitted, modified, adapted, performed, displayed, published, or sold in whole or part, without prior written permission from the American Physical Society.”]
Tennessine

Discovered by: Joint Institute for Nuclear Research, Lawrence Livermore National Laboratory, Vanderbilt University and Oak Ridge National Laboratory (2009).
What is it? A radioactive element.
Why is it important? It is the second-heaviest known element.
Haber-Bosch Process
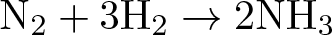
Discovered by: Fritz Haber and Carl Bosch, received the Nobel Prize in Chemistry in 1918.
What is it? An artificial nitrogen fixation process.
Why is it important? The main industrial procedure for the production of ammonia today, a milestone in industrial chemistry with deep consequences in agriculture.
Newton's First Law
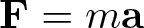
Discovered by: Isaac Newton, published in the Principia Mathematica in 1687.
What is it? Law I: Every body persists in its state of being at rest or of moving uniformly straight forward, except insofar as it is compelled to change its state by force impressed.
Why is it important? Newton's laws of motion are three physical laws that, together, laid the foundation for classical mechanics. They describe the relationship between a body and the forces acting upon it, and its motion in response to those forces.
Folic acid
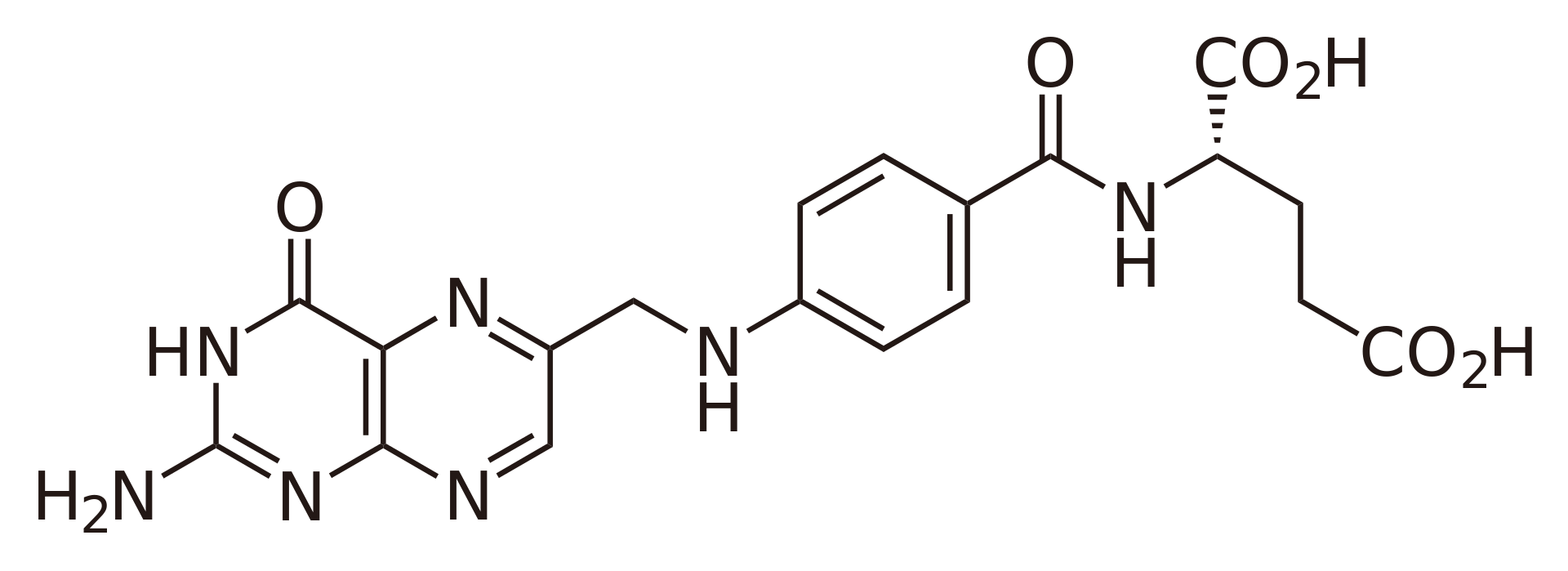
Discovered by: Lucy Wills in 1920s while researching macrocytic anaemia of pregnancy. Wills found that a nutritional factor in yeast that both prevents and cures the disorder.
What is it? aka. Vitamin B9/Wills factor. An essential building block in the production of DNA, RNA and neccessary to metabolize amino acids.
Why is it important? Deficiencies lead to a form of anemia, resulting in low redblood cell counts with associated fatigue, heart palpitations and shortness of breath. During pregnancy a lack of Folic acid can lead to neural tube defects in the baby. Most countries now fortify certain foodstuffs with the vitamin.
Black Hole Image M87*
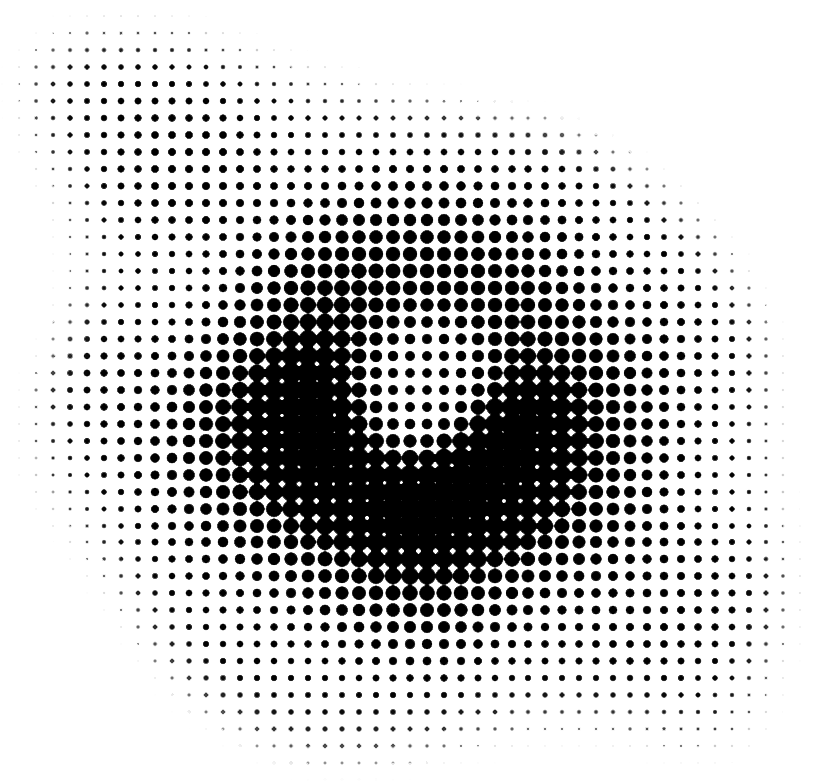
Discovered by: Event Horizon Telescope Team
What is it? A region of spacetime exhibiting gravitational acceleration so strong that nothing—no particles or even electromagnetic radiation such as light—can escape from it.
Why is it important? On 10 April 2019, the first ever direct image of a black hole and its vicinity was published, following observations made by the Event Horizon Telescope in 2017 of the supermassive black hole in Messier 87's galactic centre.
Calcium

Discovered by: First isolated in 1808 via electrolysis of its oxide by Humphry Davy.
What is it? A reactive metal that forms a dark oxide-nitride layer when exposed to air.
Why is it important? Calcium is the most abundant metal and the fifth-most abundant element in the human body.
Charles Darwin's Evolutionary Tree
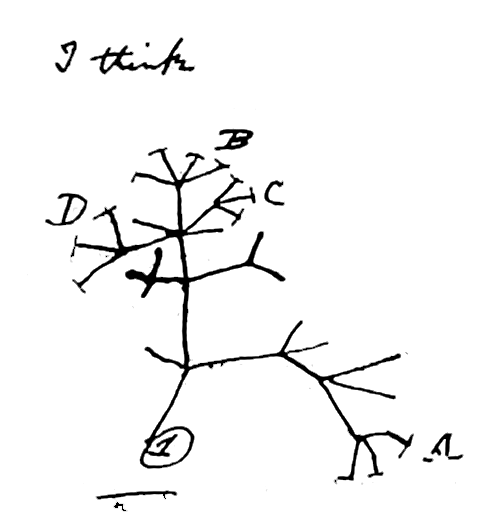
Discovered by: Charles Darwin in 1837.
What is it? Evolution is the change in the heritable characteristics of biological populations over successive generations.
Why is it important? The theory of evolution by natural selection was conceived independently by Charles Darwin and Alfred Russel Wallace in the mid-19th century as an explanation for why organisms are adapted to their physical and biological environments.
Voyager Spacecraft

Discovered by: NASA JPL.
What is it? A pair of space probes launched by NASA in 1977, as part of the Voyager program to study the outer Solar System and interstellar space beyond the Sun's heliosphere.
Why is it important? At a distance of 162 AU (24 billion km) from Earth as of November 2023, it is the most distant human-made object from Earth. "Voyager did things no one predicted, found scenes no one expected, and promises to outlive its inventors. Like a great painting or an abiding institution, it has acquired an existence of its own, a destiny beyond the grasp of its handlers." - Stephen J. Pyne
eV
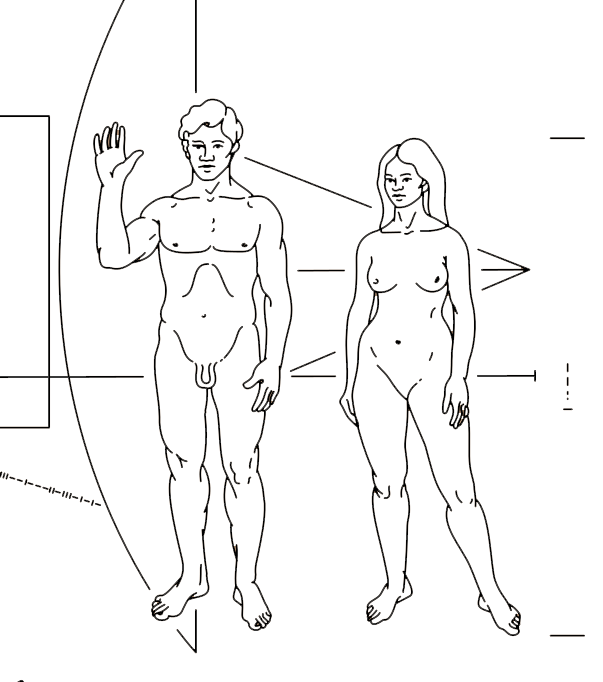
Discovered by: Image credit: Carl Sagan, Frank Drake, artwork by Linda Salzman Sagan.
What is it? eV is the female counterpart to Atom in the Voyagers sculpture.
Why is it important? The more I researched the great discoveries for the etchings, the more I realised a 'simple celebration' of our scientific past was lacking. While the telescope mirror of Atom's face had become the focal point of the male sculpture, I began to see a void for the female. The plinth, as a section of spacetime, seemed the perfect medium to carry this message. As spacetime is warped by mass I decided to show her presence in the space beside him through the granite. On first glance I hope people will wonder why there is only a man in a sculpture dedicated to humanity. The woman visible only through her effect on space, and the discoveries inscribed on his skin. The contributions of women to scientific endeavour have often been overlooked, at times actively discouraged, and even occasionally stolen. I hope the composition of this sculpture can help highlight the past, and add its weight to the wheels of change as we continue our voyage of discovery.
Atomic Mass Constant

Discovered by: Named for John Dalton who proposed to use the (still unknown) atomic mass of the lightest atom, hydrogen, as the natural unit of atomic mass.
What is it? It is defined precisely as 1/12 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest.
Why is it important? A fundamental constant in physics.
Thomson Scattering
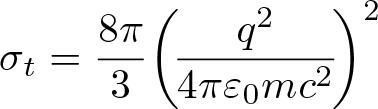
Discovered by: J. J. Thomson.
What is it? The elastic scattering of electromagnetic radiation by a free charged particle, as described by classical electromagnetism.
Why is it important? The cosmic microwave background is linearly polarized as a result of Thomson scattering, as measured by DASI and more recent experiments.
Neptunium

Discovered by: Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940.
What is it? A radioactive actinide metal, neptunium is the first transuranic element.
Why is it important? While neptunium itself has no commercial uses at present, it is used as a precursor for the formation of plutonium-238, and in radioisotope thermal generators to provide electricity for spacecraft. Neptunium has also been used in detectors of high-energy neutrons.
Gibbs Free Energy
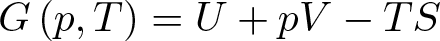
Discovered by: Josiah Willard Gibbs in the 1870s.
What is it? A thermodynamic potential that can be used to calculate the maximum of reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.
Why is it important? Also used to predict whether a chemical reaction will occur spontaneously at constant temperature and pressure.
Diethyl Ether Anaesthetic
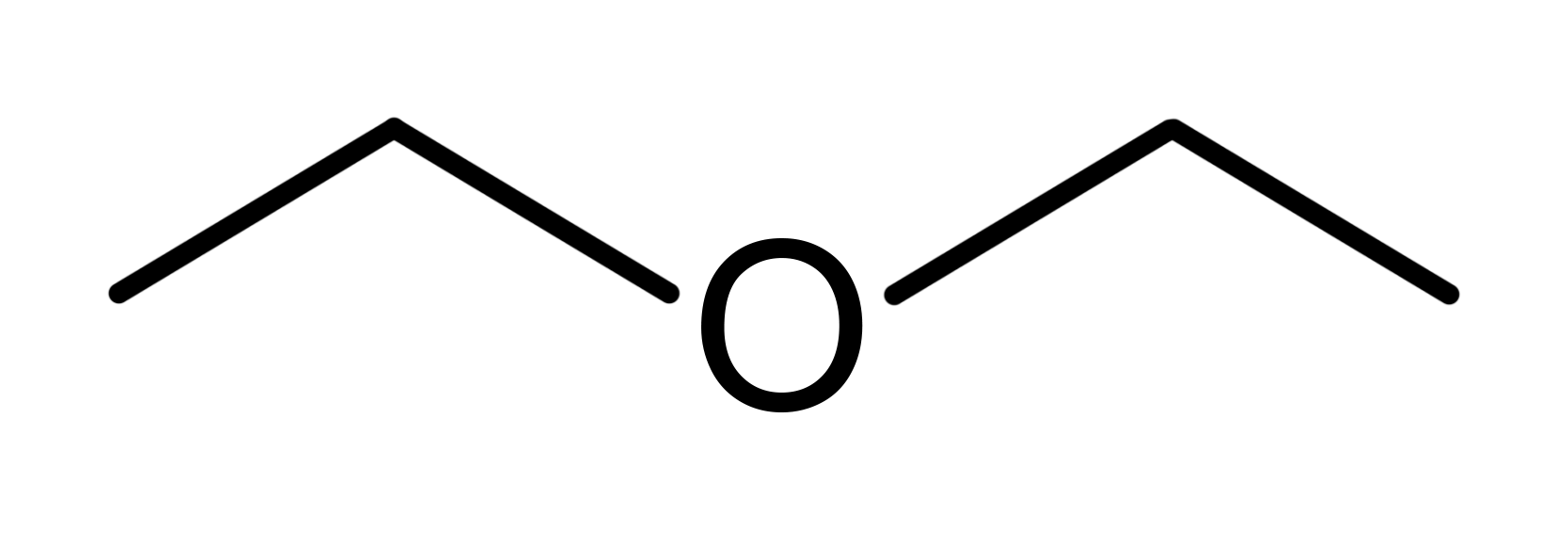
Discovered by: William T. G. Morton in a tooth extraction demonstration but its effects likely known previous to this.
What is it? A colorless, highly volatile flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines.
Why is it important? An early anesthetic with more favorable properties than chloroform.
Hawking Radiation Temperature
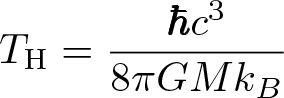
Discovered by: Stephen Hawking in 1974.
What is it? Black-body radiation that is predicted to be released by black holes, due to quantum effects near the event horizon.
Why is it important? Hawking radiation reduces the mass and rotation energy of black holes and is therefore also known as black hole evaporation. Because of this, black holes that do not gain mass through other means are expected to shrink and ultimately vanish.
Hamilton's Quaternion
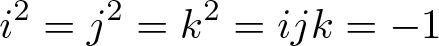
Discovered by: William Rowan Hamilton in 1843.
What is it? In mathematics, the quaternions are a number system that extends the complex numbers.
Why is it important? Quaternions find uses in both pure and applied mathematics, in particular for calculations involving three-dimensional rotations such as in three-dimensional computer graphics, computer vision, and crystallographic texture analysis.
Gravitational Waves - Radiated Power
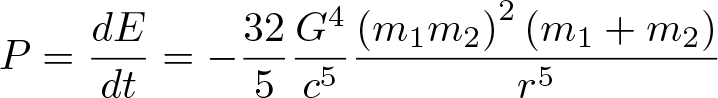
Discovered by: Proposed by Henri Poincaré in 1905, predicted in 1916 by Albert Einstein, and observed in 2016 by the LIGO + Virgo Scientific Collaboration.
What is it? The ripples in spacetime generated by orbiting black holes carry energy away from the pair making them spiral towards each other and eventually merge.
Why is it important? The discovery opens the door to a new form of astronomy to collect observational data about sources of detectable gravitational waves such as binary star systems composed of white dwarfs, neutron stars, and black holes; and events such as supernovae, and the formation of the early universe shortly after the Big Bang. In 2017, the Nobel Prize in Physics was awarded to Rainer Weiss, Kip Thorne and Barry Barish for their role in the direct detection of gravitational waves.
Pulsars
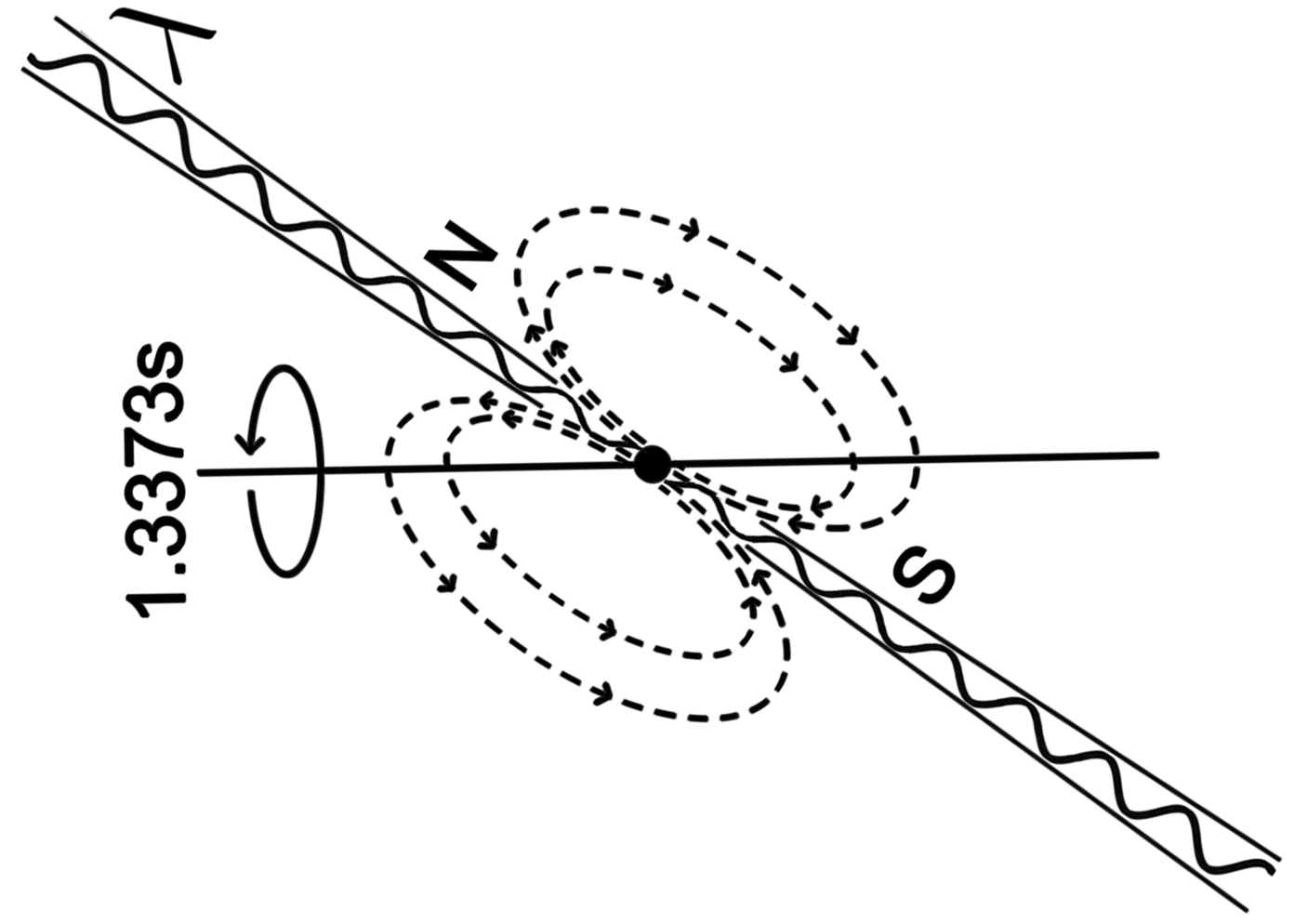
Discovered by: Jocelyn Bell Burnell in 1967.
What is it? A highly magnetized rotating neutron star that emits beams of electromagnetic radiation out of its magnetic poles.
Why is it important? The periods of pulsars make them very useful tools for astronomers. Observations of a pulsar in a binary neutron star system were used to indirectly confirm the existence of gravitational radiation. The first extrasolar planets were discovered around a pulsar, PSR B1257+12 in 1992. In 1983, certain types of pulsars were detected that, at that time, exceeded the accuracy of atomic clocks in keeping time.
Meiosis
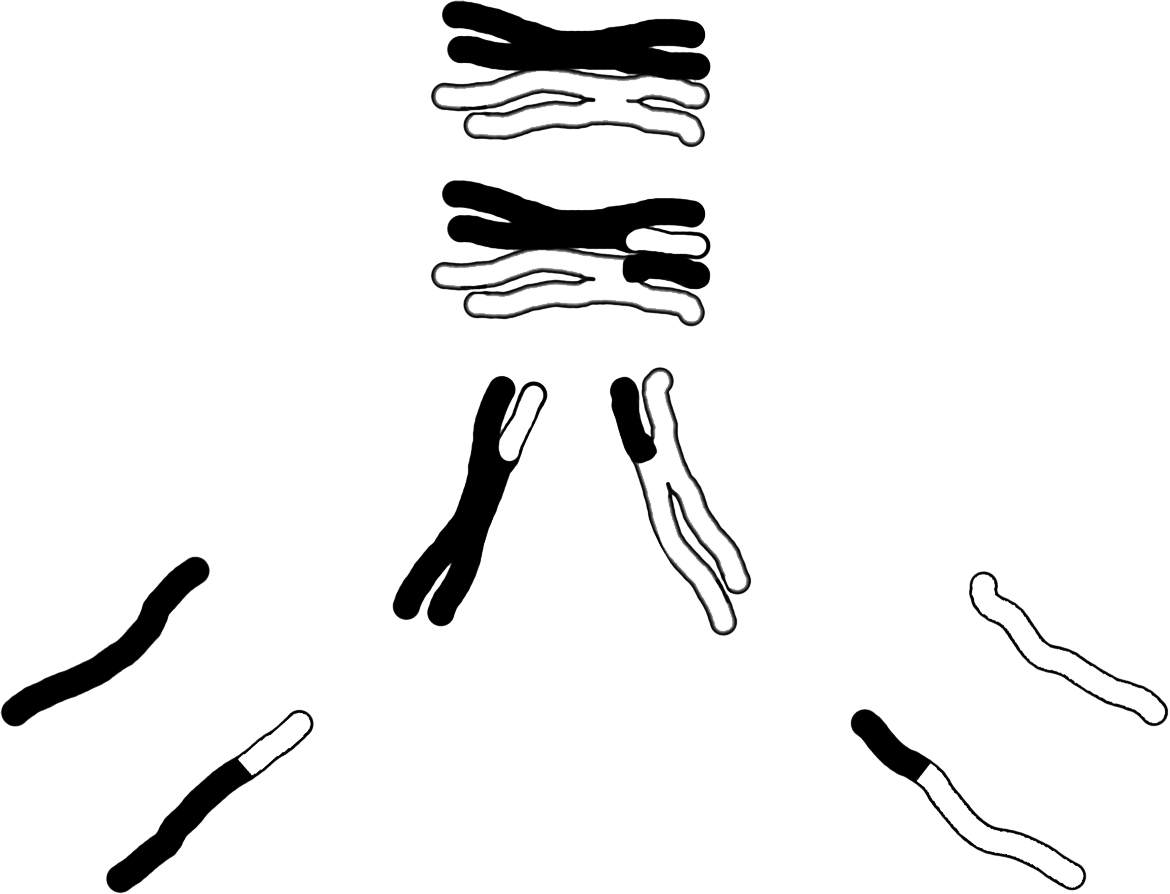
Discovered by: Oscar Hertwig in 1876.
What is it? A special type of cell division that reduces the chromosome number by half, creating four haploid cells, each genetically distinct from the parent cell that gave rise to them.
Why is it important? This process occurs in all sexually reproducing single-celled and multicellular eukaryotes, including animals, plants, and fungi. Meiotic cell divisions are an essential process during oogenesis and spermatogenesis.
Fermi Coupling Constant
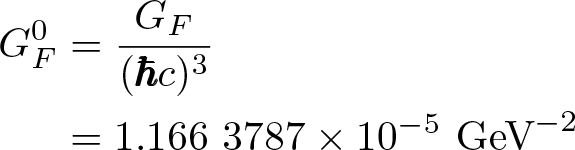
Discovered by: Enrico Fermi in 1933.
What is it? A coupling constant related to the Fermi interaction. The most precise experimental determination of the Fermi constant comes from measurements of the muon lifetime, which is inversely proportional to the square of G_F.
Why is it important? A fundamental constant of physics. This interaction explains beta decay of a neutron by direct coupling of a neutron with an electron, a neutrino (later determined to be an antineutrino) and a proton.
Compton Scattering
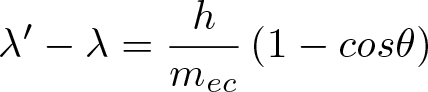
Discovered by: Arthur Holly Compton, received the 1927 Nobel Prize in Physics.
What is it? The inelastic scattering of a photon by a charged particle, usually an electron, part of the energy of the photon is transferred to the recoiling electron.
Why is it important? The mechanism by which gamma and x-rays interact with biologic tissues ie. radiotherapy.
Lanthanum
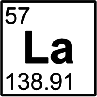
Discovered by: Carl Gustaf Mosander, who separated out two other oxides from ceria (now shown to be a mixture) which he named lanthana and didymia.
What is it? It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air.
Why is it important? Lanthanum compounds have numerous applications as catalysts, additives in glass, carbon arc lamps for studio lights and projectors, ignition elements in lighters and torches, electron cathodes, scintillators, gas tungsten arc welding electrodes.
Boron

Discovered by: Boron was not recognized as an element until it was isolated by Sir Humphry Davy and by Joseph Louis Gay-Lussac and Louis Jacques Thénard.
What is it? a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust.
Why is it important? Boron is primarily used in chemical compounds. About half of all production consumed globally is an additive in fiberglass for insulation and structural materials. The next leading use is in polymers and ceramics in high-strength, lightweight structural and heat-resistant materials.
Human Immunodeficiency Virus

Discovered by: In 1983 by two separate research groups, one led by Robert Gallo and the second by Françoise Barré-Sinoussi and Luc Montagnier, the three shared the 2008 Nobel Prize in Physiology or Medicine for the discovery.
What is it? They are two species of Lentivirus (a subgroup of retrovirus) that causes HIV infection and over time acquired immunodeficiency syndrome (AIDS).
Why is it important? HIV is a retrovirus that primarily infects components of the human immune system such as CD4+ T cells, macrophages and dendritic cells. It directly and indirectly destroys CD4+ T cells.
Pauli Exclusion Principle
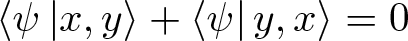
Discovered by: Wolfgang Pauli, received the Nobel Prize in Physics in 1945 for his discovery of a new law of Nature.
What is it? The quantum mechanical principle that states that two or more identical fermions (particles with half-integer spin) cannot occupy the same quantum state within a quantum system simultaneously.
Why is it important? One particularly important consequence of the principle is the elaborate electron shell structure of atoms and the way atoms share electrons, explaining the variety of chemical elements and their chemical combinations.
Proton Emission
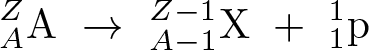
Discovered by: Sigurd Hofmann.
What is it? A rare type of radioactive decay in which a proton is ejected from a nucleus.
Why is it important? The study of proton emission has aided the understanding of nuclear deformation, masses, and structure, and it is a pure example of quantum tunneling.
Gold

Discovered by: The earliest recorded metal employed by humans appears to be gold, which can be found free or "native". Small amounts of natural gold have been found in Spanish caves used during the late Paleolithic period, c.40,000 BC.
What is it? It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal in pure form.
Why is it important? It is one of the least reactive chemical elements and is solid under standard conditions. It is a precious metal that has been used for coinage, jewelry, and other arts throughout recorded history. In the past, a gold standard was often implemented as a monetary policy.
Standard Model
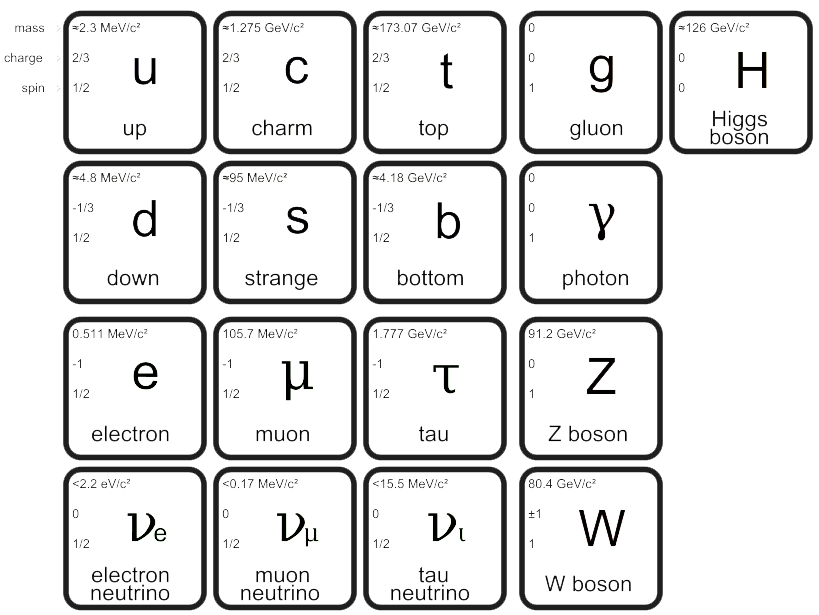
Discovered by: Many scientists.
What is it? The theory describing three of the four known fundamental forces (the electromagnetic, weak, and strong interactions, and not including the gravitational force) in the universe, as well as classifying all known elementary particles.
Why is it important? It is currently our best description of the known universe. [Image - MissMJ, Public Domain, https://commons.wikimedia.org/w/index.php?curid=4286964]
Sodium

Discovered by: First isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide.
What is it? It is a soft, silvery-white, highly reactive metal.
Why is it important? Sodium is an essential element for all animals and some plants. Sodium ions are the major cation in the extracellular fluid. Sodium and chlorine are the most common dissolved elements by weight in the oceans.
Nobelium
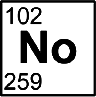
Discovered by: Joint Institute of Nuclear Research at Dubna in 1966.
What is it? A radioactive metal, it is the tenth transuranic element and is the penultimate member of the actinide series.
Why is it important? The chemical properties of nobelium are not completely known.
Completing the Square
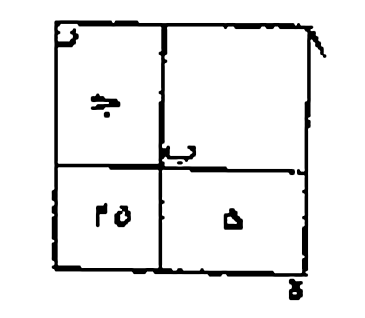
Discovered by: Muhammad ibn Musa al-Khwarizmi.
What is it? Used to solve quadratic equations.
Why is it important? In mathematics, completing the square is often applied in any computation involving quadratic polynomials.
Titanium

Discovered by: Titanium was discovered in 1791 by the clergyman and geologist William Gregor as an inclusion of a mineral in Cornwall, Great Britain.
What is it? A metal with a silver color, low density, high strength, and resistance to corrosion.
Why is it important? Titanium can be alloyed with iron, aluminium, vanadium, and molybdenum, among other elements, to produce strong, lightweight alloys.
Double-slit Experiment
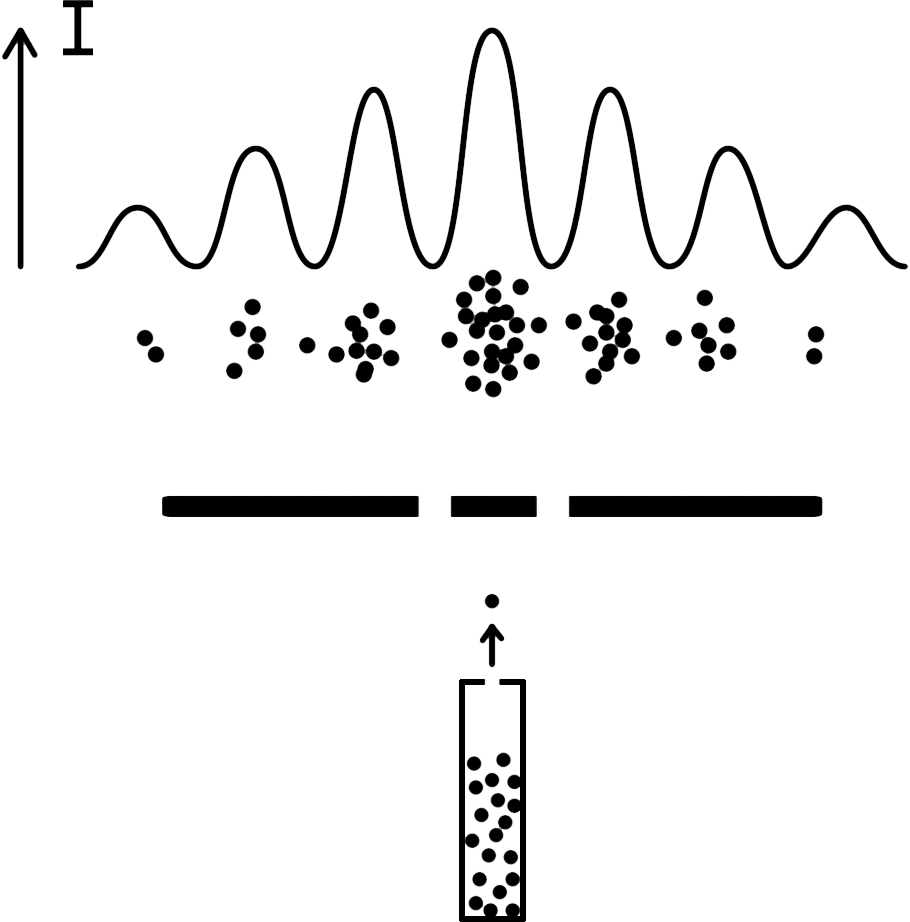
Discovered by: First performed with light by Thomas Young in 1801. In 1927, Clinton Davisson and Lester Germer demonstrated that electrons show the same behavior, which was later extended to atoms and molecules.
What is it? A demonstration that light and matter can display characteristics of both classically defined waves and particles.
Why is it important? It displays the fundamentally probabilistic nature of quantum mechanical phenomena.
Cadmium

Discovered by: It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate.
What is it? A soft, silvery-white metal, it is chemically similar to the two other stable metals in group 12, zinc and mercury.
Why is it important? Cadmium was used for a long time as a corrosion-resistant plating on steel, and cadmium compounds are used as red, orange, and yellow pigments, to color glass, and to stabilize plastic. Cadmium use is generally decreasing because it is toxic.
Estradiol
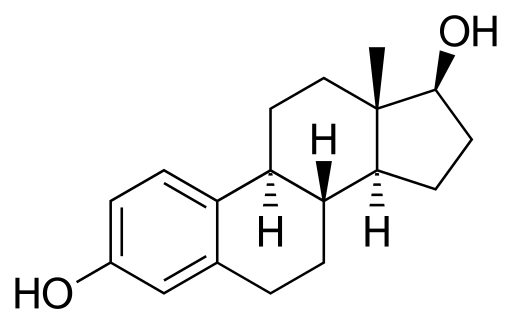
Discovered by: Edgar Allen and Edward A. Doisy in 1923.
What is it? An estrogen steroid hormone and the major female sex hormone.
Why is it important? It is involved in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips, and a female-associated pattern of fat distribution and is important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus, and vagina during puberty, adulthood, and pregnancy. It also has important effects in many other tissues including bone, fat, skin, liver, and the brain.
Pi
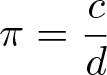
Discovered by: In Babylon, a clay tablet dated 1900–1600 BC has a geometrical statement that, by implication, treats 'pi' as 25/8 = 3.125.
What is it? The number 'pi' is a mathematical constant. Originally defined as the ratio of a circle's circumference to its diameter, it now has various equivalent definitions and appears in many formulas in all areas of mathematics and physics. It is approximately equal to 3.14159.
Why is it important? Found in many formulae in trigonometry and geometry, especially those concerning circles, ellipses, and spheres. In more modern mathematical analysis, the number is instead defined using the spectral properties of the real number system, as an eigenvalue or a period, without any reference to geometry. It appears therefore in areas of mathematics and the sciences having little to do with the geometry of circles, such as number theory and statistics, as well as in almost all areas of physics. The ubiquity of 'pi' makes it one of the most widely known mathematical constants both inside and outside the scientific community.
General Relativity
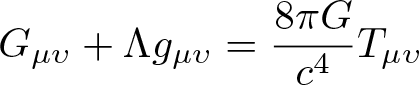
Discovered by: Albert Einstein in 1915.
What is it? A unified description of gravity as a geometric property of space and time, or spacetime. In particular, the curvature of spacetime is directly related to the energy and momentum of whatever matter and radiation are present.
Why is it important? The modern understanding of gravity where mass and energy warp the fabric of spacetime. It describes the universe on grand scales including black holes, gravitational waves and accounts for the gravitational time-dilation experienced by GPS satellites.
Copernicium
Discovered by: Gesellschaft für Schwerionenforschung (1996).
What is it? A radioactive metal.
Why is it important? The most stable known isotope, copernicium-285, has a half-life of approximately 30 seconds.
Newton's Universal Law of Gravitation
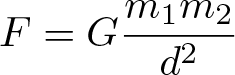
Discovered by: Isaac Newton between 1665-1687.
What is it? Every particle attracts every other particle in the universe with a force which is directly proportional to the product of their masses and inversely proportional to the square of the distance between their centers.
Why is it important? In this simple equation he describes the movements of falling apples, stars and galaxies.
Telegraphy

Discovered by: Pavel Schilling built the first electrical telegraph. Samuel Morse contributed to the invention of a single-wire telegraph system based on European telegraphs. He was a co-developer of Morse code and helped to develop the commercial use of telegraphy.
What is it? The long-distance transmission of textual messages where the sender uses symbolic codes, known to the recipient, rather than a physical exchange of an object bearing the message.
Why is it important? The telegraph freed communication from the time constraints of postal mail and revolutionized the global economy and society. [Image - Public Domain https://commons.wikimedia.org/w/index.php?curid=402251]
Stokes-Raman Scattering
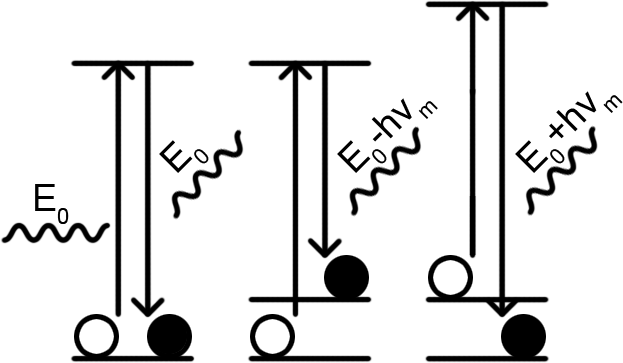
Discovered by: C. V. Raman, awarded the 1930 Nobel Prize for Physics.
What is it? The inelastic scattering of photons by matter, meaning that there is an exchange of energy and a change in the light's direction.
Why is it important? The effect is exploited by chemists and physicists to gain information about materials for a variety of purposes by performing various forms of Raman spectroscopy.
Induced Radioactivity
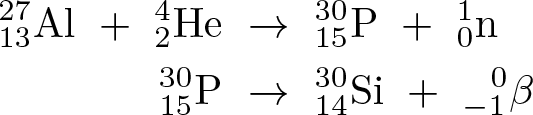
Discovered by: The husband and wife team of Irène Joliot-Curie and Frédéric Joliot-Curie discovered induced radioactivity in 1934, and they shared the 1935 Nobel Prize in Chemistry for this discovery.
What is it? When light elements, such as boron and aluminium, are bombarded with alpha-particles they continue to emit radiation after the alpha-source is removed. The original isotope has been coverted into an unstable radioactive form.
Why is it important? The most common form of induced radioactivity is neutron activation. This is seen in fission and fusion nuclear reactors, due to the high number of neutrons released in the reactions. This leads to the reactor components becoming radioactive. The phenomenon can also be used to transmute heavy elements into even heavier ones leading to the discovery of the synthetic elements.
Charge-Coupled Device
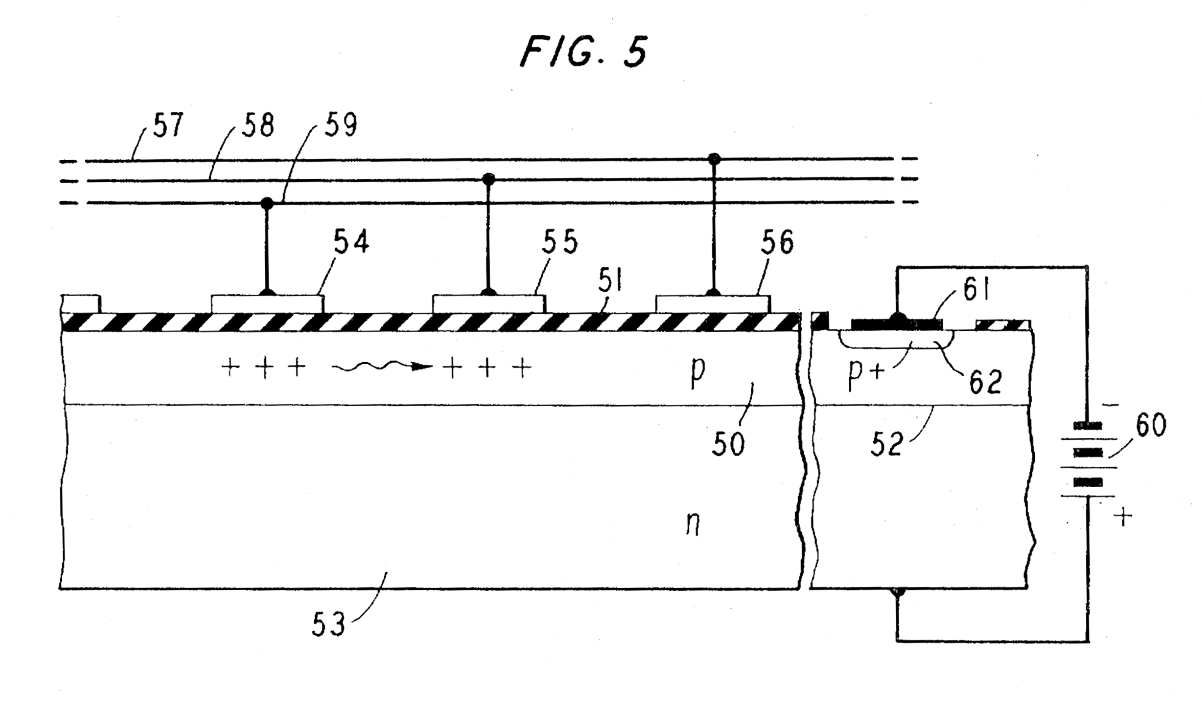
Discovered by: Willard Boyle and George E. Smith in the late 1960s.
What is it? A device for the movement of electrical charge, usually from within the device to an area where the charge can be manipulated, such as conversion into a digital value.
Why is it important? CCD is a major technology for digital imaging. In a CCD image sensor, pixels are represented by p-doped metal–oxide–semiconductor (MOS) capacitors. [Image - U.S. Patent 3,792,322]
Antimony
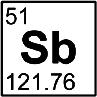
Discovered by: An artifact, said to be part of a vase, made of antimony dating to about 3000 BC was found at Telloh, Chaldea (part of present-day Iraq).
What is it? A brittle, silver-white, shiny metalloid.
Why is it important? The largest applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, bullets, and plain bearings. Antimony is used as a dopant in semiconductor devices.
Coulomb's Constant
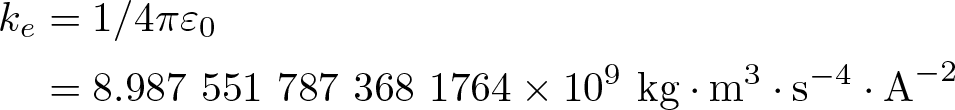
Discovered by: The "international coulomb" based on laboratory specifications for its measurement was introduced by the International Electrical Congress in 1908.
What is it? Also called the electric force constant, or the electrostatic constant is a proportionality constant in electrodynamics equations. The value of this constant is dependent upon the medium that the charged objects are immersed in. Shown here in SI units, in the case of vacuum.
Why is it important? A fundamental constant in physics.
Snell's Law
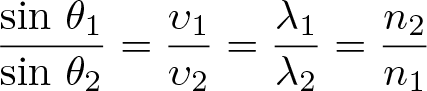
Discovered by: First accurately described by Ibn Sahl in 984 the law was named after Willebrord Snellius who derived an equivalent form in 1621.
What is it? A formula used to describe the relationship between the angles of incidence and refraction, when referring to light or other waves passing through a boundary between two different isotropic media, such as water, glass, or air.
Why is it important? Used in optics to predict how lenses will affect light rays.
Direct Methods (Crystallography)
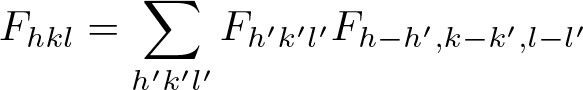
Discovered by: Herbert A. Hauptman and Jerome Karle, awarded the 1985 Nobel prize in Chemistry.
What is it? A family of methods for estimating the phases of the Fourier transform of the scattering density from the corresponding magnitudes.
Why is it important? Direct methods are the preferred method for phasing crystals of small molecules having up to 1000 atoms in the asymmetric unit.
Hemoglobin
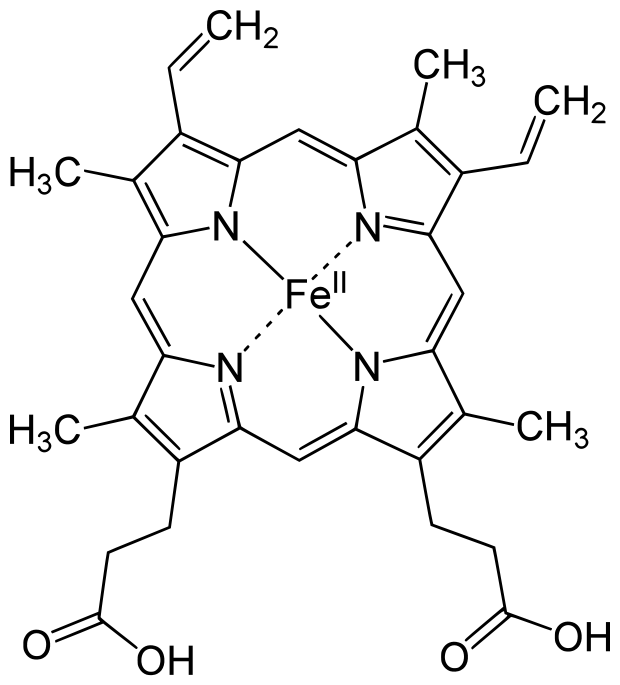
Discovered by: Max Perutz won the 1962 Nobel Prize for chemistry for his work determining the molecular structure of hemoglobin.
What is it? The iron-containing oxygen-transport protein present in erythrocytes (red blood cells) of almost all vertebrates.
Why is it important? In mammals, hemoglobin makes up about 96% of a red blood cell's dry weight (excluding water). Hemoglobin has an oxygen-binding capacity of 1.34 mL of O2 per gram,[6] which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood plasma alone.
Ribonucleic Acid - RNA
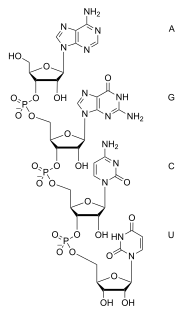
Discovered by: Nucleic acids were discovered in 1868 by Friedrich Miescher, who called the material 'nuclein' since it was found in the nucleus.
What is it? A polymeric molecule that is essential for most biological functions, either by performing the function itself or by forming a template for the production of proteins. RNA and deoxyribonucleic acid (DNA) are nucleic acids.
Why is it important? The nucleic acids constitute one of the four major macromolecules essential for all known forms of life.
Planck's Law
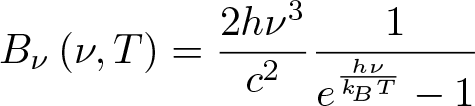
Discovered by: Max Planck in 1900.
What is it? Describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature T.
Why is it important? It is a pioneering result of modern physics and quantum theory. The most striking incarnation is its fit with the measured Cosmic Microwave Background.
Heisenberg's Uncertainty Principle
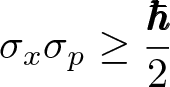
Discovered by: Werner Heisenberg, awarded the Nobel Prize in Physics in 1932.
What is it? The more precisely the position of a particle is determined, the less precisely its momentum can be known, and vice versa.
Why is it important? Shows there is a fundamental limit to the precision with which certain pairs of physical properties of a particle can be known.
First Fundamental Theorem of Calculus
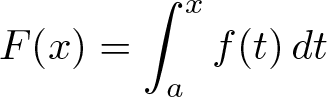
Discovered by: The first published statement and proof of a rudimentary form of the fundamental theorem, strongly geometric in character, was by James Gregory (1638–1675). Isaac Barrow (1630–1677) proved a more generalized version of the theorem, while his student Isaac Newton (1642–1727) completed the development of the surrounding mathematical theory. Gottfried Leibniz (1646–1716) systematized the knowledge into a calculus for infinitesimal quantities and introduced the notation used today.
What is it? a theorem that links the concept of differentiating a function (calculating its slopes, or rate of change at each time) with the concept of integrating a function (calculating the area under its graph, or the cumulative effect of small contributions).
Why is it important? Calculus is immensely important and sees widespread uses in science, engineering, and social science.
Hydrogen
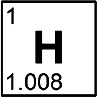
Discovered by: In 1766–1781, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former".
What is it? A chemical element; it has symbol H and atomic number 1. It is the lightest element, and at standard conditions it is a gas.
Why is it important? Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.
Nitrogen

Discovered by: Attributed to the Scottish physician Daniel Rutherford in 1772, who called it noxious air.
What is it? At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas.
Why is it important? N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate.
Arrhenius Equation
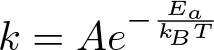
Discovered by: Svante Arrhenius, received the Nobel Prize for Chemistry in 1903.
What is it? A formula for the temperature dependence of reaction rates.
Why is it important? It can be used to model the temperature variation of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally-induced processes/reactions.
Special Relativity
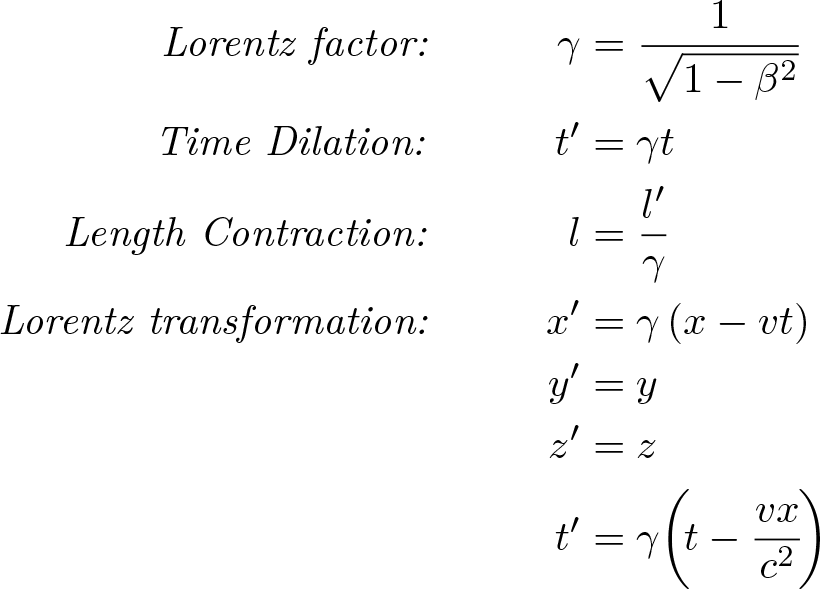
Discovered by: Albert Einstein and Hendrik Lorentz.
What is it? The laws of physics are invariant (i.e. identical) in all inertial frames of reference (i.e. non-accelerating frames of reference). The speed of light in a vacuum is the same for all observers, regardless of the motion of the light source or observer.
Why is it important? Einstein's theory corrects mechanics to handle situations involving motions at a significant fraction of the speed of light, known as relativistic velocities. It paved the way for the later theory of General Relativity. Hendrik Lorentz derived the transformation equations which formed the basis of the special relativity theory.
Starmap
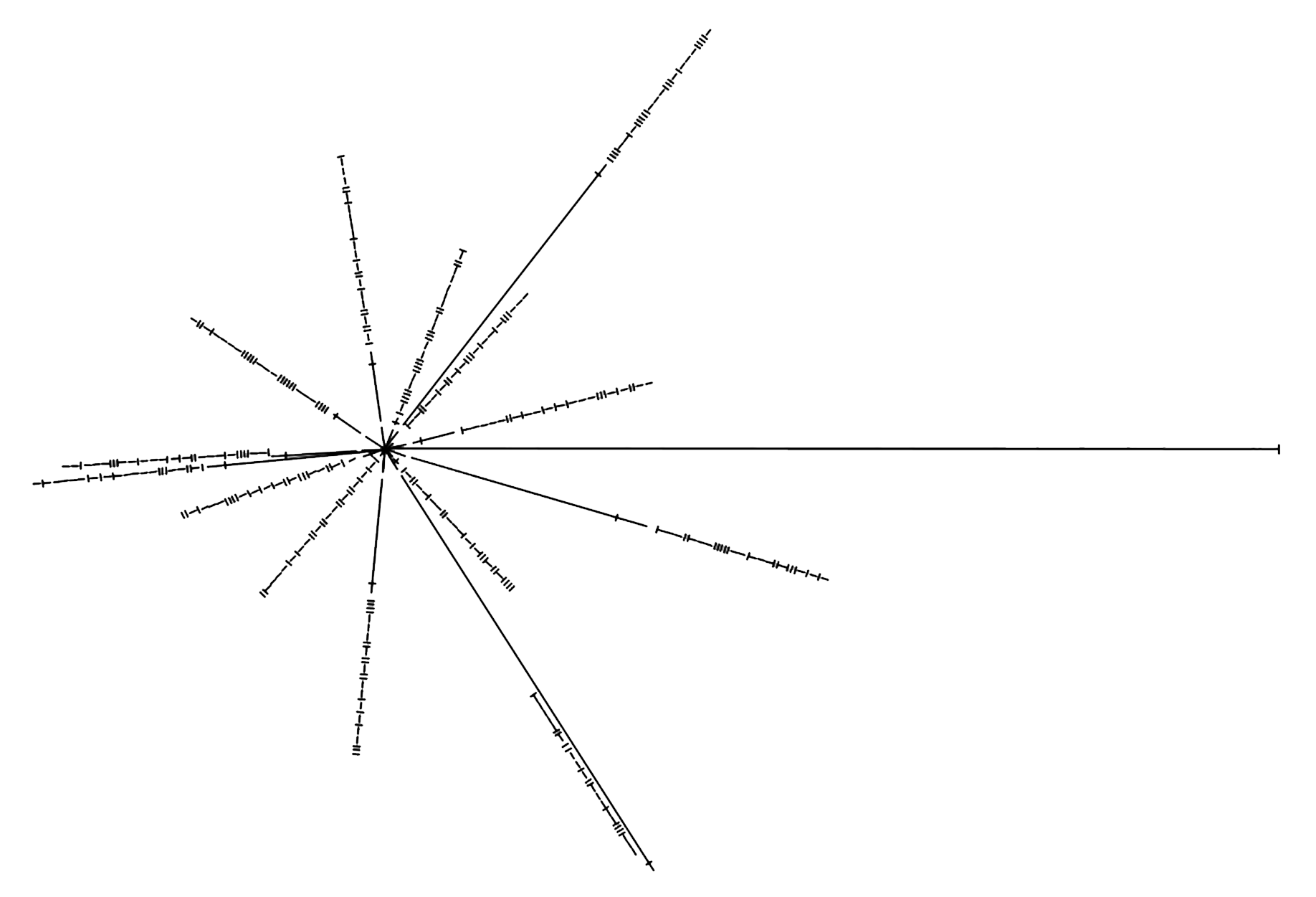
Discovered by: Carl Sagan, Frank Drake and Linda Salzman Sagan.
What is it? This diagram defines the location of our sun using 14 pulsars with established orientation from our sun. The binary code defines the frequency of the pulses.
Why is it important? Should extraterrestrial life ever encounter the Pioneer or Voyager spacecraft, this map would help them locate us in the galaxy.
Dirac Equation
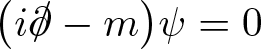
Discovered by: Paul Dirac, received the Nobel Prize in Physics in 1933.
What is it? A relativistic wave equation, it describes all spin-1/2 massive particles such as electrons and quarks.
Why is it important? Often described as 'The most beautiful equation', it brings together the two cornerstones of modern physics: quantum mechanics and relativity. It also implied the existence of a new form of matter, antimatter, previously unsuspected and unobserved and which was experimentally confirmed by the discovery of the positron. This accomplishment has been described as fully on a par with the works of Newton, Maxwell, and Einstein.
BCS Theory
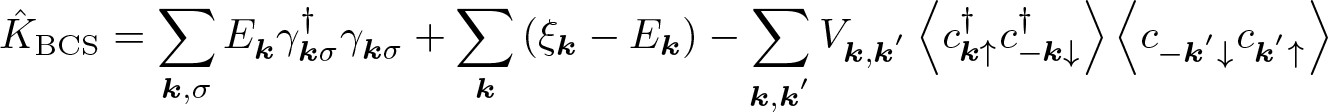
Discovered by: John Bardeen, Leon Cooper and John Robert Schrieffer, received the Nobel Prize in Physics for this theory in 1972.
What is it? The first microscopic theory of superconductivity since Heike Kamerlingh Onnes's 1911 discovery. The theory describes superconductivity as a microscopic effect caused by a condensation of Cooper pairs.
Why is it important? BCS is able to give an approximation for the quantum-mechanical many-body state of the system of electrons inside the metal.
Pythagorean Theorem
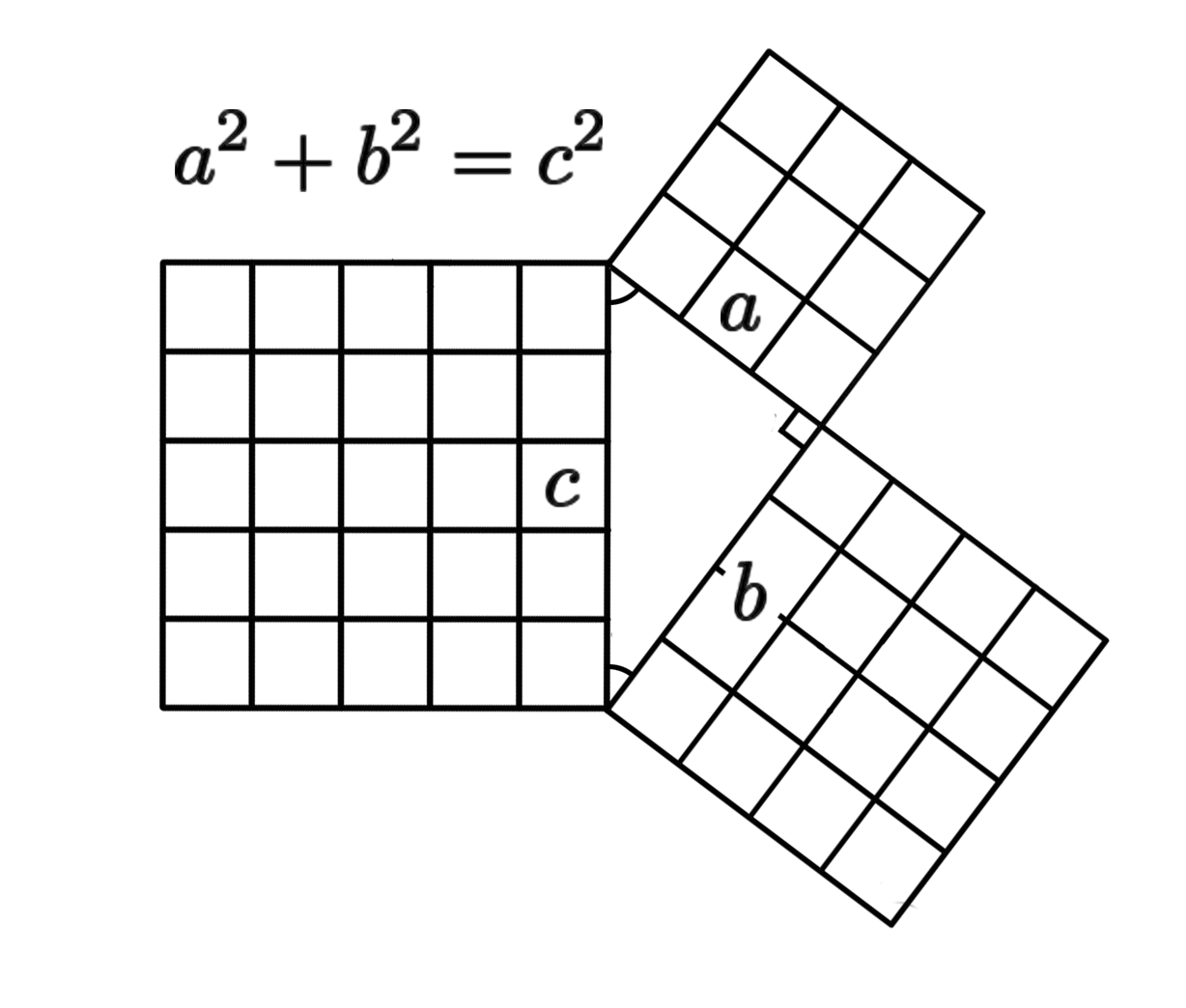
Discovered by: Named after Pythagoras but likely known before.
What is it? A fundamental relation in Euclidean geometry among the three sides of a right triangle. It states that the area of the square whose side is the hypotenuse (the side opposite the right angle) is equal to the sum of the areas of the squares on the other two sides.
Why is it important? A simple and well known mathematical formula, it appears in many different areas from geometry, trigonometry, complex numbers etc.
Yttrium

Discovered by: Friedrich Wöhler is credited with first isolating the metal in 1828 by reacting a volatile chloride that he believed to be yttrium chloride with potassium.
What is it? It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element".
Why is it important? The most important present-day use of yttrium is as a component of phosphors, especially those used in LEDs. Historically, it was once widely used in the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and tracing various materials to enhance their properties.
Higgs Lagrangian
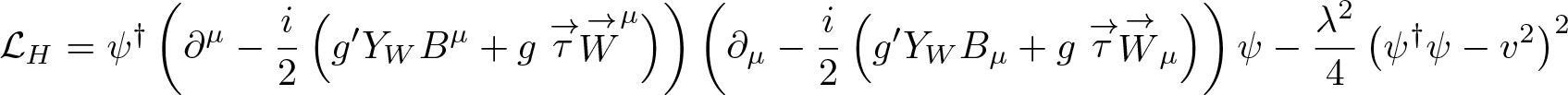
Discovered by: Independently by three teams Robert Brout and François Englert; by Peter Higgs; and by Gerald Guralnik, Carl Richard Hagen, and Tom Kibble. Peter Higgs and François Englert were awarded the 2013 Nobel Prize in Physics.
What is it? The Lagrangian density equation for the Higgs field.
Why is it important? In the Standard Model of particle physics, the Higgs mechanism is essential to explain the generation mechanism of the property "mass" for gauge bosons.
Retina
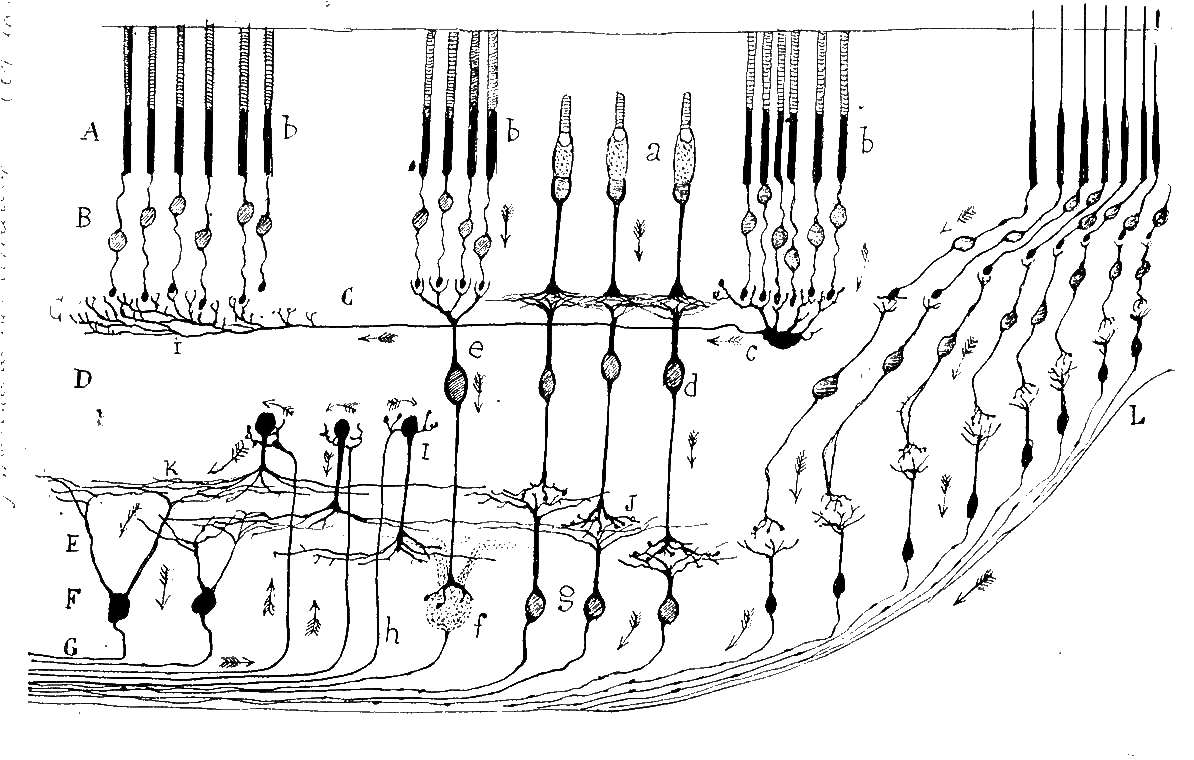
Discovered by: Santiago Ramón y Cajal published the first major characterization of retinal neurons in Retina der Wirbelthiere (The Retina of Vertebrates).
What is it? The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs.
Why is it important? The optics of the eye create a focused two-dimensional image of the visual world on the retina, which translates that image into electrical neural impulses to the brain to create visual perception, the retina serving a function analogous to that of the film or image sensor in a camera. [Image - Santiago Ramón y Cajal. Courtesy of the \href{http://www.cajal.csic.es/ingles/index.html}{Cajal Institute}. CSIC. Spain. http://www.cajal.csic.es/LegadoCajal/index.php/FondoDibujosCientificos/25938]
Planck-Einstein Relation
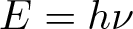
Discovered by: Max Planck, received the Nobel Prize in Physics in 1918.
What is it? The formula states that the energy of a photon, E, is proportional to its frequency, v.
Why is it important? Integral to quantum mechanics, it accounts for the quantised nature of light, and plays a key role in understanding phenomena such as the photoelectric effect, and Planck's law of black body radiation.
Cosmic Microwave Background
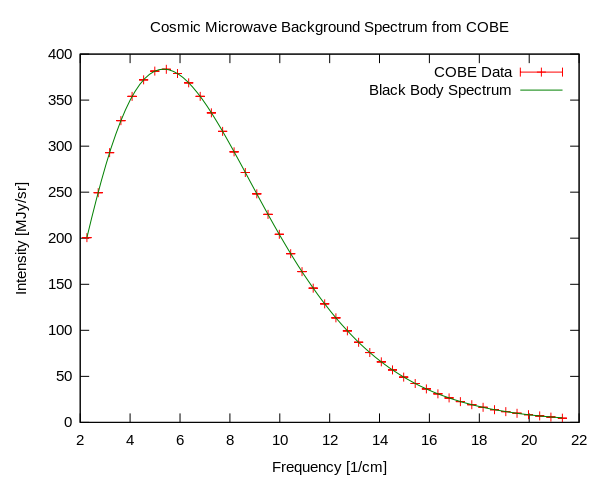
Discovered by: Arno Allan Penzias and Robert Woodrow Wilson, awarded the 1978 Nobel Prize in Physics.
What is it? Electromagnetic radiation as a remnant from an early stage of the universe.
Why is it important? CMB is landmark evidence of the Big Bang origin of the universe. [Image - Quantum Doughnut, Public Domain, https://commons.wikimedia.org/w/index.php?curid=12958270]
Energy-Mass Equivalence
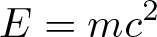
Discovered by: Albert Einstein proposed it in 1905.
What is it? The equation states that anything with mass has an equivalent energy, equal to that mass multiplied by the speed of light squared, and vice-versa.
Why is it important? The basic physics underpinning nuclear power from fission and fusion as energy is released from lost mass.
Venera 7
Discovered by: OKB-301, also called Lavochkin Research and Production Association, is a Russian aerospace company.
What is it? A Soviet spacecraft, part of the Venera series of probes to Venus.
Why is it important? When it landed on the Venusian surface on 15 December 1970, it became the first spacecraft to soft land on another planet and the first to transmit data from there back to Earth. [Image - USSR Post]
Argon

Discovered by: First isolated from air in 1894 by Lord Rayleigh and Sir William Ramsay at University College London by removing oxygen, carbon dioxide, water, and nitrogen from a sample of clean air.
What is it? The most abundant noble gas, comprising 0.00015% of Earth's crust.
Why is it important? Mostly used as an inert shielding gas in welding and other high-temperature industrial processes where ordinarily unreactive substances become reactive; for example, an argon atmosphere is used in graphite electric furnaces to prevent the graphite from burning.
Born Rule
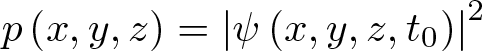
Discovered by: Walther Bothe and Max Born, awarded the Nobel Prize in Physics in 1954 for this and other work.
What is it? The probability of finding a particle at a given point is proportional to the square of the magnitude of the particle's wavefunction at that point.
Why is it important? The Born rule is one of the key principles of quantum mechanics.
Chemical Structure
Discovered by: August Kekulé in 1857–58.
What is it? Kekulé was the principal formulator of the theory of chemical structure (1857–58). This theory proceeds from the idea of atomic valence, especially the tetravalence of carbon (which Kekulé announced late in 1857) and the ability of carbon atoms to link to each other (announced in a paper published in May 1858), to the determination of the bonding order of all of the atoms in a molecule.
Why is it important? An early step in the formalisation of chemistry. His most famous work was on the structure of benzene. [Image - Friedrich August Kekulé von Stradonitz, Public Domain, https://commons.wikimedia.org/w/index.php?curid=11198902]
Polymer Chemistry
Discovered by: Hermann Staudinger, received the 1953 Nobel Prize in Chemistry.
What is it? The polymerisation reaction of isoprene monomers to polyisoprene.
Why is it important? In a landmark paper published in 1920, he proposed that rubber and other polymers such as starch, cellulose and proteins are long chains of short repeating molecular units linked by covalent bonds.
Electric Motor
Discovered by: Many scientists and engineers, but often credited to Michael Faraday. This later design is by Nikola Tesla.
What is it? An electrical machine that converts electrical energy into mechanical energy.
Why is it important? Used in a wide range of modern technology including vacuum cleaners, hard drives, cars and industrial equipment. [Image - US381968A]
Young's Modulus
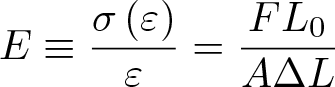
Discovered by: The concept was developed in 1727 by Leonhard Euler, Giordano Riccati performed the first experiments in 1782, and was characterised by Thomas Young in 1807.
What is it? A mechanical property that measures the stiffness of a solid material.
Why is it important? Young's modulus enables the calculation of the change in the dimension of a bar made of an isotropic elastic material under tensile or compressive loads.
Beta-plus Decay
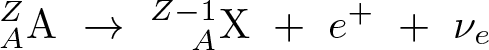
Discovered by: Frédéric Joliot-Curie and Irène Joliot-Curie.
What is it? A proton is converted into a neutron, a positron, and an electron neutrino.
Why is it important? Beta-plus decay can only happen inside nuclei when the absolute value of the binding energy of the daughter nucleus is greater than that of the parent nucleus, i.e., the daughter nucleus is a lower-energy state.
Graphene
Discovered by: Andre Geim and Konstantin Novoselov, were awarded the 2010 Nobel Prize in Physics.
What is it? An allotrope of carbon in the form of a single layer of atoms in a two-dimensional hexagonal lattice in which one atom forms each vertex. It is the basic structural element of other allotropes, including graphite, charcoal, carbon nanotubes and fullerenes.
Why is it important? In proportion to its thickness, it is about 100 times stronger than the strongest steel, yet its density is dramatically lower. It conducts heat and electricity very efficiently and is nearly transparent.
Cholesterol
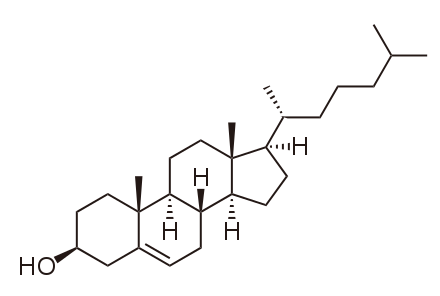
Discovered by: François Poulletier de la Salle first identified cholesterol in solid form in gallstones in 1769.
What is it? A type of lipid known as a steroid alcohol.
Why is it important? Cholesterol also serves as a precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. It composes about 30\% of all animal cell membranes and modulates membrane fluidity over the range of physiological temperatures.
N-(4-methoxybenzylidene)-4-butylaniline (MBBA)
Discovered by: Hans Kelker.
What is it? MBBA is an organic compound often used as a liquid crystal, a state of matter which has properties between those of conventional liquids and those of solid crystals.
Why is it important? One of the most popular subjects of liquid crystal research. Lead to the development of further chemicals used in liquid crystal displays.
Pyrimethamine
Discovered by: Gertrude Elion in 1952.
What is it? A medication used with leucovorin to treat the parasite diseases toxoplasmosis and cystoisosporiasis.
Why is it important? Also used with dapsone as a second-line option to prevent Pneumocystis jiroveci pneumonia in people with HIV/AIDS. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.
Noether's Theorem
Discovered by: Emmy Noether in 1915.
What is it? Every different symmetry of a physical thing has a conservation law.
Why is it important? For example, if you hit two marbles together on the table, it would be the same as hitting them together on the floor; location does not matter as long as they are hit together in the same way. Here, the conserved quantity is momentum. Often called "one of the most important mathematical theorems ever proved in guiding the development of modern physics".
Vostok 6

Discovered by: OKB-1, the largest company of the Russian space industry.
What is it? The spacecraft that Valentina Tereshkova flew into space.
Why is it important? The first woman in space launched on the 16th June 1963 and spent nearly 3 days in orbit. [Image - Luc van den Abeelen, Public Domain]
Alpha Decay
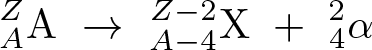
Discovered by: Ernest Rutherford.
What is it? A type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two.
Why is it important? Approximately 99\% of the helium produced on Earth is the result of the alpha decay of underground deposits of minerals containing uranium or thorium. The helium is brought to the surface as a by-product of natural gas production.
Newton's Third Law
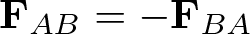
Discovered by: Isaac Newton, published in the Principia Mathematica in 1687.
What is it? Law II: The alteration of motion is ever proportional to the motive force impress'd; and is made in the direction of the right line in which that force is impress'd.
Why is it important? Newton's laws of motion are three physical laws that, together, laid the foundation for classical mechanics. They describe the relationship between a body and the forces acting upon it, and its motion in response to those forces.
Bose-Einstein Condensate Transition
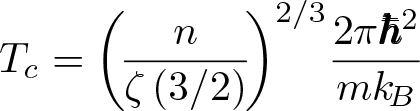
Discovered by: Satyendra Nath Bose and Albert Einstein.
What is it? A state of matter of a dilute gas of bosons cooled to temperatures very close to 0K. Einstein proposed that cooling bosonic atoms to a very low temperature would cause them to fall (or "condense") into the lowest accessible quantum state, resulting in a new form of matter.
Why is it important? Eric Cornell, Carl Wieman and Wolfgang Ketterle produced the first BECs in the lab and received the 2001 Nobel Prize in Physics. These early studies founded the field of ultracold atoms, and hundreds of research groups around the world now routinely produce BECs of dilute atomic vapors in their labs.
Oxygen
Discovered by: Polish alchemist, philosopher, and physician Michael Sendivogius c1600.
What is it? It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.
Why is it important? Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. All plants, animals, and fungi need oxygen for cellular respiration, which extracts energy by the reaction of oxygen with molecules derived from food and produces carbon dioxide as a waste product.
Taylor Series
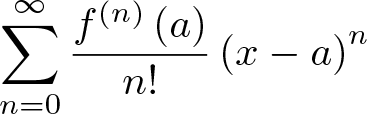
Discovered by: The general method for constructing these series was provided by Brook Taylor.
What is it? A representation of a function as an infinite sum of terms that are calculated from the values of the function's derivatives at a single point.
Why is it important? The polynomial formed by taking some initial terms of the Taylor series provides an approximation of the original function which can be evaluated.
Landau-Lifshitz-Gilbert Equation
Discovered by: Lev Landau, Evgeny Lifshitz and T. L. Gilbert.
What is it? A differential equation describing the precessional motion of magnetization M in a solid.
Why is it important? The various forms of the equation are commonly used in micromagnetics to model the effects of a magnetic field on ferromagnetic materials. In particular it can be used to model the time domain behavior of magnetic elements due to a magnetic field.
Logarithms
Discovered by: Logarithms were introduced by John Napier in 1614 as a means to simplify calculations.
What is it? In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a given number 'x' is the exponent to which another fixed number, the base 'b', must be raised, to produce that number 'x'.
Why is it important? Using logarithm tables, tedious multi-digit multiplication steps can be replaced by table look-ups and simpler addition. They were rapidly adopted by navigators, scientists, engineers, surveyors and others to perform high-accuracy computations more easily.
Cepheid Variables as Standard Candles
Discovered by: Henrietta Swan Leavitt in 1908.
What is it? A type of star that pulsates radially, varying in both diameter and temperature and producing changes in brightness with a well-defined stable period and amplitude.
Why is it important? The strong correllation between a Cepheid variable's luminosity and pulsation period established Cepheids as important indicators of cosmic benchmarks for scaling galactic and extragalactic distances. aka. Standard candles. [Image - Leavitt, Henrietta S; Pickering, Edward C, Public Domain, https://commons.wikimedia.org/w/index.php?curid=34747012]
Hubble's Law
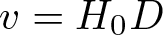
Discovered by: Edwin Hubble, Alexander Friedmann and Georges Lemaître.
What is it? This Doppler shift-measured velocity of various galaxies receding from the Earth is approximately proportional to their distance from the Earth for galaxies up to a few hundred megaparsecs away.
Why is it important? Hubble's law is considered the first observational basis for the expansion of the universe and today serves as one of the pieces of evidence most often cited in support of the Big Bang model.
Planck Constant
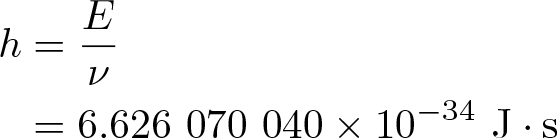
Discovered by: Max Planck in 1900.
What is it? The quantum of electromagnetic action, which relates the energy carried by a photon to its frequency.
Why is it important? A fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a matter wave equals the Planck constant divided by the associated particle momentum.
Lutetium
Discovered by: Independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James.
What is it? It is a silvery white metal, which resists corrosion in dry air, but not in moist air.
Why is it important? Sometimes used in metal alloys and as a catalyst in various chemical reactions.
Inner Sphere Electron Transfer
Discovered by: Henry Taube, awarded the 1983 Nobel Prize in Chemistry.
What is it? The Creutz–Taube ion, created by Carol Creutz, has been heavily studied in an effort to understand the intimate details of inner sphere electron transfer.
Why is it important? A redox chemical reaction that proceeds via a covalent linkage—a strong electronic interaction—between the oxidant and the reductant reactants.
Bakelite
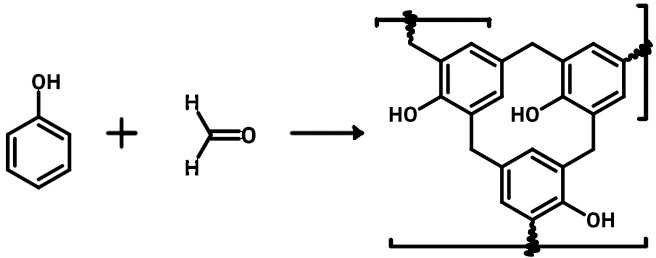
Discovered by: Leo Baekeland in 1907.
What is it? A thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde.
Why is it important? One of the first plastics made from synthetic components. Bakelite was used for its electrical nonconductivity and heat-resistant properties in electrical insulators, radio and telephone casings and such diverse products as kitchenware, jewelry, pipe stems, children's toys, and firearms.
Ginzburg-Landau Theory
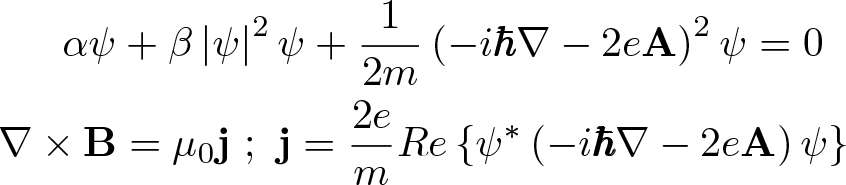
Discovered by: Vitaly Ginzburg and Lev Landau.
What is it? A mathematical physical theory used to describe superconductivity.
Why is it important? A phenomenological model which could describe type-I superconductors without examining their microscopic properties.
Atom Theories
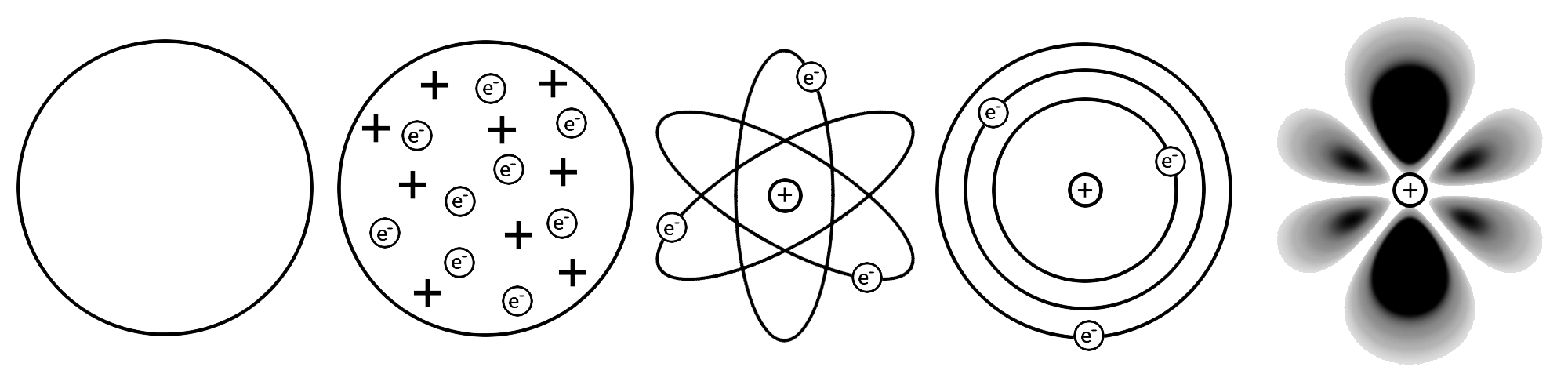
Discovered by: John Dalton, J. J. Thomson, Ernest Rutherford, Niels Bohr, Erwin Schrödinger.
What is it? The progress of the scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. Dalton's atomic spheres, Thomson's plum pudding, Rutherford's planetary model, Bohr's quantized electron levels, Shrodinger's electron wavefunction.
Why is it important? Shows our changing understanding of the stuff that makes up matter itself.
HeLa Cell Line
Discovered by: George Otto Gey is the cell biologist at Johns Hopkins Hospital who is credited with propagating the HeLa cell line.
What is it? The first immortalized human cell line taken from Henrietta Lacks in 1951. The cell culture known as HeLa is used for medical research.
Why is it important? The cells from Lacks's cancerous cervical tumor were taken without her knowledge, which was common practice in the United States at the time. They could be cultured rapidly and used in a wide variety of biomedical research; from testing vaccines, cancer research, effects of radiation, and gene mapping to name a few. As much as 50 million tons of her cells have been cultured from the first sample.
Weinberg Angle
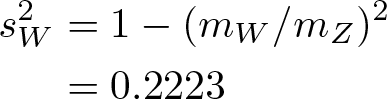
Discovered by: Steven Weinberg and Mohammad Abdus Salam.
What is it? A parameter in the Weinberg–Salam theory of the electroweak interaction, part of the Standard Model of particle physics.
Why is it important? It is the angle by which spontaneous symmetry breaking rotates the original W0 and B0 vector boson plane, producing as a result the Z0 boson, and the photon.
Schrodinger Equation
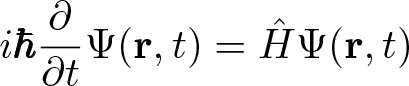
Discovered by: Erwin Schrodinger, received the 1933 Nobel Prize in Physics.
What is it? A linear partial differential equation that describes the wave function or state function of a quantum-mechanical system.
Why is it important? One of the cornerstones of modern quantum mechanics, the Schrodinger equation is a mathematical equation that describes the evolution over time of a physical system in which quantum effects, such as wave-particle duality, are significant.
Olefin Metathesis
Discovered by: Yves Chauvin, Robert H. Grubbs and Richard R. Schrock, collectively awarded the 2005 Nobel Prize in Chemistry.
What is it? An organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds.
Why is it important? Used in many industrial applications from detergent production to pharmaceutical drugs.
Vitamin B12
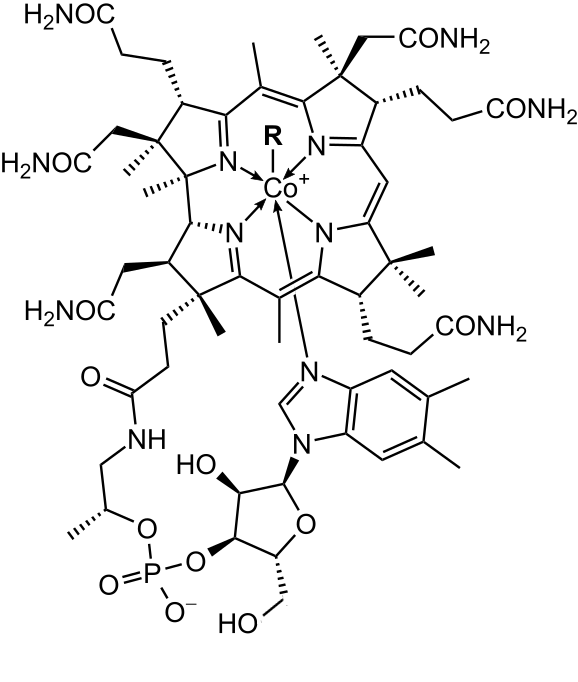
Discovered by: Dorothy Hodgkin, a British chemist who developed protein crystallography and discovered the structure of vitamin B12 for which she won the Nobel Prize in Chemistry in 1964.
What is it? One of eight B vitamins; it is the largest and most structurally complex vitamin.
Why is it important? It is involved in the metabolism of every cell of the human body: it is a cofactor in DNA synthesis, and in both fatty acid and amino acid metabolism. It is particularly important in the normal functioning of the nervous system via its role in the synthesis of myelin, and in the maturation of developing red blood cells in the bone marrow. [Image - Ymwang42, Public Domain, https://commons.wikimedia.org/w/index.php?curid=9461097]
Steam Turbine
Discovered by: Charles Algernon Parsons in 1884.
What is it? The steam turbine is a form of heat engine that derives much of its improvement in thermodynamic efficiency from the use of multiple stages in the expansion of the steam, which results in a closer approach to the ideal reversible expansion process.
Why is it important? Because the turbine generates rotary motion, it is particularly suited to be used to drive an electrical generator—about 85\% of all electricity generation in the United States in the year 2014 was by use of steam turbines. [Image - US775634A]
Roentgenium

Discovered by: Gesellschaft für Schwerionenforschung (1994).
What is it? A radioactive metal.
Why is it important? It is extremely radioactive and can only be created in a laboratory. The most stable known isotope, roentgenium-282, has a half-life of 120 seconds, although the unconfirmed roentgenium-286 may have a longer half-life of about 10.7 minutes.
Barium

Discovered by: First isolated by electrolysis of molten barium salts in 1808 by Sir Humphry Davy in England.
What is it? A soft, silvery alkaline earth metal.
Why is it important? Barium has few industrial applications. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. In a purer form it is used as X-ray radiocontrast agents for imaging the human gastrointestinal tract.
Einstein's Theory of Brownian Motion
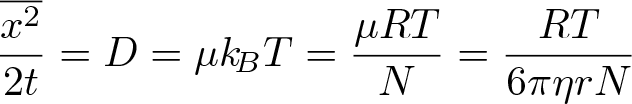
Discovered by: Albert Einstein.
What is it? Einstein showed that the motion Brown had observed was a result of the pollen being moved by individual water molecules.
Why is it important? This explanation of Brownian motion served as convincing evidence that atoms and molecules exist, and was further verified experimentally by Jean Perrin in 1908. Perrin was awarded the Nobel Prize in Physics in 1926.
Quasicrystal
Discovered by: Dan Shechtman, awarded the 2011 Nobel Prize in Chemistry.
What is it? A crystalline structure that is ordered but not periodic.
Why is it important? While crystals, according to the classical crystallographic restriction theorem, can possess only two-, three-, four-, and six-fold rotational symmetries, the Bragg diffraction pattern of quasicrystals shows sharp peaks with other symmetry orders — for instance, five-fold.
Curium
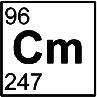
Discovered by: Glenn T. Seaborg, Ralph A. James, Albert Ghiorso in 1944.
What is it? Curium is a hard, dense, silvery metal with a high melting and boiling point for an actinide.
Why is it important? Curium is used in making heavier actinides and the 238Pu radionuclide for power sources in artificial cardiac pacemakers and RTGs for spacecraft. It served as the α-source in the alpha particle X-ray spectrometers of several space probes, including the Sojourner, Spirit, Opportunity, and Curiosity Mars rovers and the Philae lander on comet 67P/Churyumov–Gerasimenko, to analyze the composition and structure of the surface.