The Second Law of Thermodynamics
Discovered by: James Clerk Maxwell, Rudolf Clausius, Nicolas Léonard Sadi Carnot, among others.
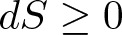
What is it? Heat cannot spontaneously flow from a colder location to a hotter location. The total entropy of an isolated system can only increase over time. It can remain constant in ideal cases where the system is in a steady state (equilibrium) or undergoing a reversible process. The increase in entropy accounts for the irreversibility of natural processes, and the asymmetry between future and past.
Why is it important? The laws of thermodynamics are important fundamental laws in physics and they are applicable in other natural sciences. They define physical quantities (temperature, energy, and entropy) that characterize thermodynamic systems at thermal equilibrium. The laws describe how these quantities behave under various circumstances, and preclude the possibility of certain phenomena (such as perpetual motion).